Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta-analysis
- PMID: 28152545
- PMCID: PMC5355921
- DOI: 10.1038/bjc.2017.12
Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta-analysis
Abstract
Background: Colorectal cancer (CRC) is common and associated with significant mortality. Current screening methods for CRC lack patient compliance. microRNAs (miRNAs), identified in body fluids, are negative regulators of gene expression and are dysregulated in many cancers, including CRC. This paper summarises studies identifying blood-based miRNAs dysregulated in CRC compared with healthy controls in an attempt to evaluate their use as a screening tool for the diagnosis of CRC.
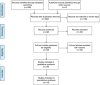
Methods: A search of electronic databases (PubMed and EMBASE) and grey literature was performed between January 2002 and April 2016. Studies reporting plasma or serum miRNAs in the diagnosis of CRC compared with healthy controls were selected. Patient demographics, type of patient sample (serum or plasma), method of miRNA detection, type of normalisation, and the number of significantly dysregulated miRNAs identified were recorded. Statistical evaluation of dysregulated miRNAs using sensitivity, specificity, and area under the curve (AUC) was performed.
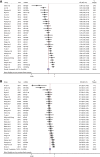
Results: Thirty-four studies investigating plasma or serum miRNAs in the diagnosis of CRC were included. A total of 31 miRNAs were found to be either upregulated (n=17) or downregulated (n=14) in CRC cases as compared with controls. Fourteen studies identified panels of ⩾2 dysregulated miRNAs. The highest AUC, 0.943, was identified using a panel of 4 miRNAs with 83.3% sensitivity and 93.1% specificity. Meta-analysis of studies identifying a single dysregulated miRNA in CRC cases compared with controls was performed. Overall sensitivity and specificity of 28 individual miRNAs in the diagnosis of CRC were 76% (95% CI 72%-80%) and 76% (95% CI 72%-80%), respectively, indicating good discriminative ability of miRNAs as biomarkers for CRC. These data did not change with sensitivity analyses.
Conclusions: Blood-based miRNAs distinguish patients with CRC from healthy controls with high sensitivity and specificity comparable to other common and invasive currently used screening methods for CRC. In future, miRNAs may be used as a relatively non-invasive blood-based marker for detection of CRC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- (2016) Test: About ColoVantage [Internet]. Questdiagnostics.com [Online]. Available at http://www.questdiagnostics.com/home/physicians/testing-services/by-test... (accessed 16 March 2016).
-
- Ahmed FE, Amed NC, Vos PW, Bonnerup C, Atkins JN, Casey M, Nuovo GJ, Naziri W, Wiley JE, Allison RR (2012) Diagnostic microRNA markers to screen for sporadic human colon cancer in blood. Cancer Genomics Proteomics 9: 179–192. - PubMed
-
- Basati G, Emami Razavi A, Abdi S, Mirzaei A (2014) Elevated level of microRNA-21 in the serum of patients with colorectal cancer. Med Oncol 31: 205. - PubMed
-
- Basati G, Razavi AE, Pakzad I, Malayeri FA (2015) Circulating levels of the miRNAs, miR-194, and miR-29b, as clinically useful biomarkers for colorectal cancer. Tumour Biol 37: 1781–1788. - PubMed
-
- Brunet Vega A, Pericay C, Moya I, Ferrer A, Dotor E, Pisa A, Casalots A, Serra-Aracil X, Oliva JC, Ruiz A, Saigi E (2013) microRNA expression profile in stage III colorectal cancer: circulating miR-18a and miR-29a as promising biomarkers. Oncol Rep 30: 320–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

