Phosphatase and tensin homolog-β-catenin signaling modulates regulatory T cells and inflammatory responses in mouse liver ischemia/reperfusion injury
- PMID: 28152578
- PMCID: PMC5449221
- DOI: 10.1002/lt.24735
Phosphatase and tensin homolog-β-catenin signaling modulates regulatory T cells and inflammatory responses in mouse liver ischemia/reperfusion injury
Abstract
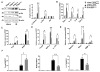
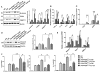
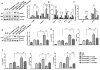
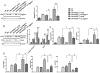
The phosphatase and tensin homolog (PTEN) deleted on chromosome 10 plays an important role in regulating T cell activation during inflammatory response. Activation of β-catenin is crucial for maintaining immune homeostasis. This study investigates the functional roles and molecular mechanisms by which PTEN-β-catenin signaling promotes regulatory T cell (Treg) induction in a mouse model of liver ischemia/reperfusion injury (IRI). We found that mice with myeloid-specific phosphatase and tensin homolog knockout (PTENM-KO ) exhibited reduced liver damage as evidenced by decreased levels of serum alanine aminotransferase, intrahepatic macrophage trafficking, and proinflammatory mediators compared with the PTEN-proficient (floxed phosphatase and tensin homolog [PTENFL/FL ]) controls. Disruption of myeloid PTEN-activated b-catenin promoted peroxisome proliferator-activated receptor gamma (PPARγ)-mediated Jagged-1/Notch signaling and induced forkhead box P3 (FOXP3)1 Tregs while inhibiting T helper 17 cells. However, blocking of Notch signaling by inhibiting γ-secretase reversed myeloid PTEN deficiency-mediated protection in ischemia/reperfusion-triggered liver inflammation with reduced FOXP3+ and increased retinoid A receptor-related orphan receptor gamma t-mediated interleukin 17A expression in ischemic livers. Moreover, knockdown of β-catenin or PPARγ in PTEN-deficient macrophages inhibited Jagged-1/Notch activation and reduced FOXP3+ Treg induction, leading to increased proinflammatory mediators in macrophage/T cell cocultures. In conclusion, our findings demonstrate that PTEN-β-catenin signaling is a novel regulator involved in modulating Treg development and provides a potential therapeutic target in liver IRI. Liver Transplantation 23 813-825 2017 AASLD.
© 2017 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Peralta C, Jimenez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. Journal of hepatology. 2013;59(5):1094–106. - PubMed
-
- Eggenhofer E, Rovira J, Sabet-Baktach M, Groell A, Scherer MN, Dahlke MH, et al. Unconventional RORgammat+ T cells drive hepatic ischemia reperfusion injury. Journal of immunology. 2013;191(1):480–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

