Bestrophin 1 and retinal disease
- PMID: 28153808
- PMCID: PMC5600499
- DOI: 10.1016/j.preteyeres.2017.01.006
Bestrophin 1 and retinal disease
Abstract
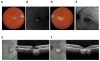
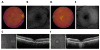
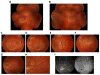
Mutations in the gene BEST1 are causally associated with as many as five clinically distinct retinal degenerative diseases, which are collectively referred to as the "bestrophinopathies". These five associated diseases are: Best vitelliform macular dystrophy, autosomal recessive bestrophinopathy, adult-onset vitelliform macular dystrophy, autosomal dominant vitreoretinochoroidopathy, and retinitis pigmentosa. The most common of these is Best vitelliform macular dystrophy. Bestrophin 1 (Best1), the protein encoded by the gene BEST1, has been the subject of a great deal of research since it was first identified nearly two decades ago. Today we know that Best1 functions as both a pentameric anion channel and a regulator of intracellular Ca2+ signaling. Best1 is an integral membrane protein which, within the eye, is uniquely expressed in the retinal pigment epithelium where it predominantly localizes to the basolateral plasma membrane. Within the brain, Best1 expression has been documented in both glial cells and astrocytes where it functions in both tonic GABA release and glutamate transport. The crystal structure of Best1 has revealed critical information about how Best1 functions as an ion channel and how Ca2+ regulates that function. Studies using animal models have led to critical insights into the physiological roles of Best1 and advances in stem cell technology have allowed for the development of patient-derived, "disease in a dish" models. In this article we review our knowledge of Best1 and discuss prospects for near-term clinical trials to test therapies for the bestrophinopathies, a currently incurable and untreatable set of diseases.
Keywords: Anion channel; Best1; Bestrophin; Maculopathy; Retinal disease; Retinal pigment epithelium.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures






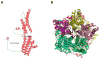
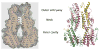
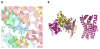




References
-
- Allikmets R, Seddon JM, Bernstein PS, Hutchinson A, Atkinson A, Sharma S, Gerrard B, Li W, Metzker ML, Wadelius C, Caskey CT, Dean M, Petrukhin K. Evaluation of the Best disease gene in patients with age-related macular degeneration and other maculopathies. Human genetics. 1999;104:449–453. - PubMed
-
- Andre S, Boukhaddaoui H, Campo B, Al-Jumaily M, Mayeux V, Greuet D, Valmier J, Scamps F. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. Journal of neurophysiology. 2003;90:3764–3773. - PubMed
-
- Arndt CF, Derambure P, Defoort-Dhellemmes S, Hache JC. Outer retinal dysfunction in patients treated with vigabatrin. Neurology. 1999;52:1201–1205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

