Function of the Plant DNA Polymerase Epsilon in Replicative Stress Sensing, a Genetic Analysis
- PMID: 28153919
- PMCID: PMC5338674
- DOI: 10.1104/pp.17.00031
Function of the Plant DNA Polymerase Epsilon in Replicative Stress Sensing, a Genetic Analysis
Abstract
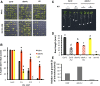
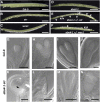
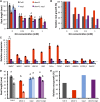
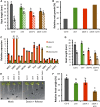
Faithful transmission of the genetic information is essential in all living organisms. DNA replication is therefore a critical step of cell proliferation, because of the potential occurrence of replication errors or DNA damage when progression of a replication fork is hampered causing replicative stress. Like other types of DNA damage, replicative stress activates the DNA damage response, a signaling cascade allowing cell cycle arrest and repair of lesions. The replicative DNA polymerase ε (Pol ε) was shown to activate the S-phase checkpoint in yeast in response to replicative stress, but whether this mechanism functions in multicellular eukaryotes remains unclear. Here, we explored the genetic interaction between Pol ε and the main elements of the DNA damage response in Arabidopsis (Arabidopsis thaliana). We found that mutations affecting the polymerase domain of Pol ε trigger ATR-dependent signaling leading to SOG1 activation, WEE1-dependent cell cycle inhibition, and tolerance to replicative stress induced by hydroxyurea, but result in enhanced sensitivity to a wide range of DNA damaging agents. Using knock-down lines, we also provide evidence for the direct role of Pol ε in replicative stress sensing. Together, our results demonstrate that the role of Pol ε in replicative stress sensing is conserved in plants, and provide, to our knowledge, the first genetic dissection of the downstream signaling events in a multicellular eukaryote.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

