The Sites of Evaporation within Leaves
- PMID: 28153921
- PMCID: PMC5338672
- DOI: 10.1104/pp.16.01605
The Sites of Evaporation within Leaves
Abstract
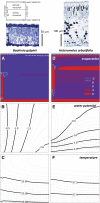
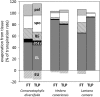
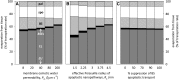
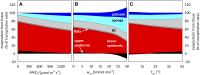
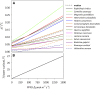
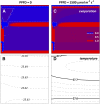
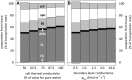
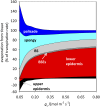
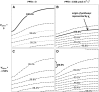
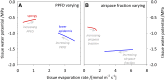
The sites of evaporation within leaves are unknown, but they have drawn attention for decades due to their perceived implications for many factors, including patterns of leaf isotopic enrichment, the maintenance of mesophyll water status, stomatal regulation, and the interpretation of measured stomatal and leaf hydraulic conductances. We used a spatially explicit model of coupled water and heat transport outside the xylem, MOFLO 2.0, to map the distribution of net evaporation across leaf tissues in relation to anatomy and environmental parameters. Our results corroborate earlier predictions that most evaporation occurs from the epidermis at low light and moderate humidity but that the mesophyll contributes substantially when the leaf center is warmed by light absorption, and more so under high humidity. We also found that the bundle sheath provides a significant minority of evaporation (15% in darkness and 18% in high light), that the vertical center of amphistomatous leaves supports net condensation, and that vertical temperature gradients caused by light absorption vary over 10-fold across species, reaching 0.3°C. We show that several hypotheses that depend on the evaporating sites require revision in light of our findings, including that experimental measurements of stomatal and hydraulic conductances should be affected directly by changes in the location of the evaporating sites. We propose a new conceptual model that accounts for mixed-phase water transport outside the xylem. These conclusions have far-reaching implications for inferences in leaf hydraulics, gas exchange, water use, and isotope physiology.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures



Comment in
-
On the Inside.Plant Physiol. 2017 Mar;173(3):1527-1528. doi: 10.1104/pp.17.00218. Plant Physiol. 2017. PMID: 28258119 Free PMC article. No abstract available.
References
-
- Barbour MM, Farquhar GD (2004) Do pathways of water movement and leaf anatomical dimensions allow development of gradients in H218O between veins and the sites of evaporation within leaves? Plant Cell Environ 27: 107–121
-
- Beerling DJ, Franks PJ (2010) The hidden cost of transpiration. Nature 464: 495–496 - PubMed
-
- Berry JA, Beerling DJ, Franks PJ (2010) Stomata: key players in the earth system, past and present. Curr Opin Plant Biol 13: 233–240 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

