Involvement of glycogen synthase kinase-3β in liver ischemic conditioning induced cardioprotection against myocardial ischemia and reperfusion injury in rats
- PMID: 28153944
- PMCID: PMC5451530
- DOI: 10.1152/japplphysiol.00862.2016
Involvement of glycogen synthase kinase-3β in liver ischemic conditioning induced cardioprotection against myocardial ischemia and reperfusion injury in rats
Abstract
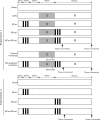
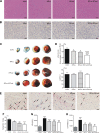
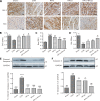
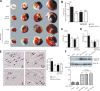
Remote ischemic conditioning has been convincingly shown to render the myocardium resistant to a subsequent more severe sustained episode of ischemia. Compared with other organs, little is known regarding the effect of transient liver ischemic conditioning. We proposed the existence of cardioprotection induced by remote liver conditioning. Male Sprague-Dawley rats were divided into sham-operated control (no further hepatic intervention) and remote liver ischemic conditioning groups. For liver ischemic conditioning, three cycles of 5 min of liver ischemia-reperfusion stimuli were conducted before-(liver preconditioning), post-myocardial ischemia (liver postconditioning), or in combination of both (liver preconditioning + liver postconditioning). Rats were exposed to 45 min of left anterior descending coronary artery occlusion, followed by 3 h of reperfusion thereafter. ECG and hemodynamics were measured throughout the experiment. The coronary artery was reoccluded at the end of reperfusion for infarct size determination. Blood samples were taken for serum lactate dehydrogenase and creatine kinase-MB test. Heart tissues were taken for apoptosis measurements and Western blotting. Our data demonstrate that liver ischemic preconditioning, postconditioning, or a combination of both, offered strong cardioprotection, as evidenced by reduction in infarct size and cardiac tissue damage, recovery of cardiac function, and inhibition of apoptosis after ischemia-reperfusion. Moreover, liver ischemic conditioning increased cardiac (not hepatic) glycogen synthase kinase-3β (GSK-3β) phosphorylation. Accordingly, inhibition of GSK-3β mimicked the cardioprotective action of liver conditioning. These results demonstrate that remote liver ischemic conditioning protected the heart against ischemia and reperfusion injury via GSK-3β-dependent cell-survival signaling pathway.NEW & NOTEWORTHY Remote ischemic conditioning protects hearts against ischemia and reperfusion (I/R) injury. However, it is unclear whether ischemic conditioning of visceral organs such as the liver, the largest metabolic organ in the body, can produce cardioprotection. This is the first study to show the cardioprotective effect of remote liver ischemic conditioning in a rat model of myocardial I/R injury. We also, for the first time, demonstrated these protective properties are associated with glycogen synthase kinase-3β-dependent cell-survival signaling pathway.
Keywords: GSK-3β; cardioprotection; liver ischemic conditioning; myocardial ischemia and reperfusion injury.
Copyright © 2017 the American Physiological Society.
Figures

Similar articles
-
Remifentanil Preconditioning Reduces Postischemic Myocardial Infarction and Improves Left Ventricular Performance via Activation of the Janus Activated Kinase-2/Signal Transducers and Activators of Transcription-3 Signal Pathway and Subsequent Inhibition of Glycogen Synthase Kinase-3β in Rats.Crit Care Med. 2016 Mar;44(3):e131-45. doi: 10.1097/CCM.0000000000001350. Crit Care Med. 2016. PMID: 26468894
-
Ischemic postconditioning mediates cardioprotection via PI3K/GSK-3β/β-catenin signaling pathway in ischemic rat myocardium.Shock. 2012 Aug;38(2):165-9. doi: 10.1097/SHK.0b013e31825b5633. Shock. 2012. PMID: 22576003
-
Ischaemic post-conditioning protects both adult and aged Sprague-Dawley rat heart from ischaemia-reperfusion injury through the phosphatidylinositol 3-kinase-AKT and glycogen synthase kinase-3beta pathways.Clin Exp Pharmacol Physiol. 2009 Aug;36(8):756-63. doi: 10.1111/j.1440-1681.2009.05148.x. Epub 2009 Jan 17. Clin Exp Pharmacol Physiol. 2009. PMID: 19207715
-
Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning.Circ Res. 2015 Feb 13;116(4):674-99. doi: 10.1161/CIRCRESAHA.116.305348. Circ Res. 2015. PMID: 25677517 Review.
-
Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning.Pharmacol Rev. 2007 Dec;59(4):418-58. doi: 10.1124/pr.107.06002. Epub 2007 Nov 29. Pharmacol Rev. 2007. PMID: 18048761 Review.
Cited by
-
Ischemic limb preconditioning-induced anti-arrhythmic effect in reperfusion-induced myocardial injury: is it mediated by the RISK or SAFE pathway?Pflugers Arch. 2022 Sep;474(9):979-991. doi: 10.1007/s00424-022-02716-5. Epub 2022 Jun 13. Pflugers Arch. 2022. PMID: 35695933
-
Aloperine Alleviates Myocardial Injury Induced by Myocardial Ischemia and Reperfusion by Activating the ERK1/2/β-catenin Signaling Pathway.Cardiovasc Drugs Ther. 2025 Jun;39(3):533-551. doi: 10.1007/s10557-024-07566-0. Epub 2024 Feb 28. Cardiovasc Drugs Ther. 2025. PMID: 38416285
-
Protective Effect of Remote Ischemic Preconditioning against Myocardial Ischemia-Reperfusion Injury in Rats and Mice: A Systematic Review and Meta-Analysis.Rev Cardiovasc Med. 2022 Dec 20;23(12):413. doi: 10.31083/j.rcm2312413. eCollection 2022 Dec. Rev Cardiovasc Med. 2022. PMID: 39076668 Free PMC article.
-
Remote ischemic preconditioning differentially attenuates post-ischemic cardiac arrhythmia in streptozotocin-induced diabetic versus nondiabetic rats.Cardiovasc Diabetol. 2017 Apr 26;16(1):57. doi: 10.1186/s12933-017-0537-3. Cardiovasc Diabetol. 2017. PMID: 28446231 Free PMC article.
-
The Multifunctional Role of KCNE2: From Cardiac Arrhythmia to Multisystem Disorders.Cells. 2024 Aug 23;13(17):1409. doi: 10.3390/cells13171409. Cells. 2024. PMID: 39272981 Free PMC article. Review.
References
-
- Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375: 727–734, 2010. doi:10.1016/S0140-6736(09)62001-8. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

