Extracellular Loops Are Essential for the Assembly and Function of Polycystin Receptor-Ion Channel Complexes
- PMID: 28154010
- PMCID: PMC5354480
- DOI: 10.1074/jbc.M116.767897
Extracellular Loops Are Essential for the Assembly and Function of Polycystin Receptor-Ion Channel Complexes
Abstract
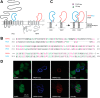
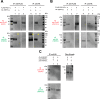
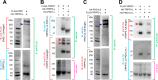
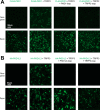
Polycystin complexes, or TRPP-PKD complexes, made of transient receptor potential channel polycystin (TRPP) and polycystic kidney disease (PKD) proteins, play key roles in coupling extracellular stimuli with intracellular Ca2+ signals. For example, the TRPP2-PKD1 complex has a crucial function in renal physiology, with mutations in either protein causing autosomal dominant polycystic kidney disease. In contrast, the TRPP3-PKD1L3 complex responds to low pH and was proposed to be a sour taste receptor candidate. It has been shown previously that the protein partners interact via association of the C-terminal or transmembrane segments, with consequences for the assembly, surface expression, and function of the polycystin complexes. However, the roles of extracellular components, especially the loops that connect the transmembrane segments, in the assembly and function of the polycystin complex are largely unknown. Here, with an immunoprecipitation method, we found that extracellular loops between the first and second transmembrane segments of TRPP2 and TRPP3 associate with the extracellular loops between the sixth and seventh transmembrane segments of PKD1 and PKD1L3, respectively. Immunofluorescence and electrophysiology data further confirm that the associations between these loops are essential for the trafficking and function of the complexes. Interestingly, most of the extracellular loops are also found to be involved in homomeric assembly. Furthermore, autosomal dominant polycystic kidney disease-associated TRPP2 mutant T448K significantly weakened TRPP2 homomeric assembly but had no obvious effect on TRPP2-PKD1 heteromeric assembly. Our results demonstrate a crucial role of these functionally underexplored extracellular loops in the assembly and function of the polycystin complexes.
Keywords: PKD; TRPP; autosomal dominant polycystic kidney disease; cell surface receptor; extracellular loop; ion channel; polycystin; protein assembly; protein-protein interaction; transient receptor potential channels (TRP channels).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Semmo M., Köttgen M., and Hofherr A. (2014) The TRPP subfamily and polycystin-1 proteins. Handb. Exp. Pharmacol. 222, 675–711 - PubMed
-
- Wu G., and Somlo S. (2000) Molecular genetics and mechanism of autosomal dominant polycystic kidney disease. Mol. Genet. Metabol. 69, 1–15 - PubMed
-
- Ramsey I. S., Delling M., and Clapham D. E. (2006) An introduction to TRP channels. Annu. Rev. Physiol. 68, 619–647 - PubMed
-
- Mochizuki T., Wu G., Hayashi T., Xenophontos S. L., Veldhuisen B., Saris J. J., Reynolds D. M., Cai Y., Gabow P. A., Pierides A., Kimberling W. J., Breuning M. H., Deltas C. C., Peters D. J., and Somlo S. (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

