Formation of α-chiral centers by asymmetric β-C(sp3)-H arylation, alkenylation, and alkynylation
- PMID: 28154075
- PMCID: PMC5480404
- DOI: 10.1126/science.aal5175
Formation of α-chiral centers by asymmetric β-C(sp3)-H arylation, alkenylation, and alkynylation
Abstract
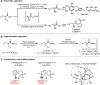
The enzymatic β-C-H hydroxylation of the feedstock chemical isobutyric acid has enabled the asymmetric synthesis of a wide variety of polyketides. The analogous transition metal-catalyzed enantioselective β-C-H functionalization of isobutyric acid-derived substrates should provide a versatile method for constructing useful building blocks with enantioenriched α-chiral centers from this abundant C-4 skeleton. However, the desymmetrization of ubiquitous isopropyl moieties by organometallic catalysts has remained an unanswered challenge. Herein, we report the design of chiral mono-protected aminomethyl oxazoline ligands that enable desymmetrization of isopropyl groups via palladium insertion into the C(sp3)-H bonds of one of the prochiral methyl groups. We detail the enantioselective β-arylation, -alkenylation, and -alkynylation of isobutyric acid/2-aminoisobutyric acid derivatives, which may serve as a platform for the construction of α-chiral centers.
Copyright © 2017, American Association for the Advancement of Science.
Figures



References
-
- Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Chem Soc Rev. 2009;38:3242–3272. - PubMed
-
- Goodhue CT, Schaeffer JR. Biotechnol Bioeng. 1971;13:203–214. - PubMed
-
- Hasegawa J, Ogura M, Hamaguchi S, Shimazaki M, Kawaharada H, Watanabe K. J Ferment Technol. 1981;59:203–208.
-
- Bode JW, Fraefel N, Muri D, Carreira EM. Angew Chem Int Ed. 2001;40:2082–2085. - PubMed
-
- Paterson I, Britton R, Delgado O, Meyer A, Poullennec KG. Angew Chem Int Ed. 2004;43:4629–4633. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

