Homer1a drives homeostatic scaling-down of excitatory synapses during sleep
- PMID: 28154077
- PMCID: PMC5382711
- DOI: 10.1126/science.aai8355
Homer1a drives homeostatic scaling-down of excitatory synapses during sleep
Abstract
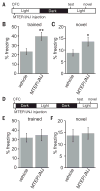
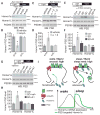
Sleep is an essential process that supports learning and memory by acting on synapses through poorly understood molecular mechanisms. Using biochemistry, proteomics, and imaging in mice, we find that during sleep, synapses undergo widespread alterations in composition and signaling, including weakening of synapses through removal and dephosphorylation of synaptic AMPA-type glutamate receptors. These changes are driven by the immediate early gene Homer1a and signaling from group I metabotropic glutamate receptors mGluR1/5. Homer1a serves as a molecular integrator of arousal and sleep need via the wake- and sleep-promoting neuromodulators, noradrenaline and adenosine, respectively. Our data suggest that homeostatic scaling-down, a global form of synaptic plasticity, is active during sleep to remodel synapses and participates in the consolidation of contextual memory.
Copyright © 2017, American Association for the Advancement of Science.
Figures


Comment in
-
Synaptic scaling in sleep.Science. 2017 Feb 3;355(6324):457. doi: 10.1126/science.aam7917. Science. 2017. PMID: 28154034 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

