Lymphocyte activation gene 3: a novel therapeutic target in chronic lymphocytic leukemia
- PMID: 28154084
- PMCID: PMC5477606
- DOI: 10.3324/haematol.2016.148965
Lymphocyte activation gene 3: a novel therapeutic target in chronic lymphocytic leukemia
Abstract
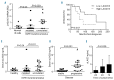
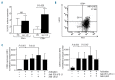
A novel therapeutic approach in cancer, attempting to stimulate host anti-tumor immunity, involves blocking of immune checkpoints. Lymphocyte activation gene 3 (LAG3) is an immune checkpoint receptor expressed on activated/exhausted T cells. When engaged by the major histocompatibility complex (MHC) class II molecules, LAG3 negatively regulates T-cell function, thereby contributing to tumor escape. Intriguingly, a soluble LAG3 variant activates both immune and malignant MHC class II-presenting cells. In the study herein, we examined the role of LAG3 in the pathogenesis of chronic lymphocytic leukemia, an MHC class II-presenting malignancy, and show that chronic lymphocytic leukemia cells express and secrete LAG3. High levels of surface and soluble LAG3 were associated with the unmutated immunoglobulin variable heavy chain leukemic subtype and a shorter median time from diagnosis to first treatment. Utilizing a mechanism mediated through MHC class II engagement, recombinant soluble LAG3-Ig fusion protein, LAG3-Fc, activated chronic lymphocytic leukemia cells, induced anti-apoptotic pathways and protected the cells from spontaneous apoptosis, effects mediated by SYK, BTK and MAPK signaling. Moreover, LAG3 blocking antibody enhanced in vitro T-cell activation. Our data suggest that soluble LAG3 promotes leukemic cell activation and anti-apoptotic effects through its engagement with MHC class II. Furthermore, MHC class II-presenting chronic lymphocytic leukemia cells may affect LAG3-presenting T cells and impose immune exhaustion on their microenvironment; hence, blocking LAG3-MHC class II interactions is a potential therapeutic target in chronic lymphocytic leukemia.
Copyright© Ferrata Storti Foundation.
Figures



References
-
- Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol. 1999; 17(1):399–408. - PubMed
-
- Moreno C, Villamor N, Colomer D, et al. Allogeneic stem-cell transplantation may overcome the adverse prognosis of unmutated VH gene in patients with chronic lymphocytic leukemia. J Clin Oncol. 2005. 20;23(15):3433–3438. - PubMed
-
- Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101(3):1087–1093. - PubMed
-
- Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118(16):4313–4320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

