pKa of Glu325 in LacY
- PMID: 28154138
- PMCID: PMC5320973
- DOI: 10.1073/pnas.1621431114
pKa of Glu325 in LacY
Abstract
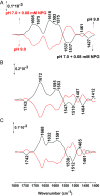
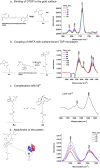
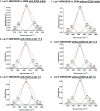
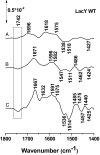
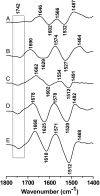
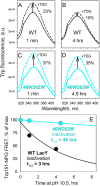
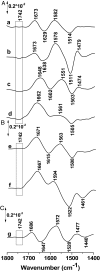
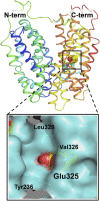
Lactose permease (LacY), a paradigm for the largest family of membrane transport proteins, catalyzes the coupled translocation of a galactoside and a H+ across the cytoplasmic membrane of Escherichia coli (galactoside/H+ symport). One of the most important aspects of the mechanism is the relationship between protonation and binding of the cargo galactopyranoside. In this regard, it has been shown that protonation is required for binding. Furthermore when galactoside affinity is measured as a function of pH, an apparent pK (pKapp) of ∼10.5 is obtained. Strikingly, when Glu325, a residue long known to be involved in coupling between H+ and sugar translocation, is replaced with a neutral side chain, the pH effect is abolished, and high-affinity binding is observed until LacY is destabilized at alkaline pH. In this paper, infrared spectroscopy is used to identify Glu325 in situ. Moreover, it is demonstrated that this residue exhibits a pKa of 10.5 ± 0.1 that is insensitive to the presence of galactopyranoside. Thus, it is apparent that protonation of Glu325 specifically is required for effective sugar binding to LacY.
Keywords: lactose permease; membrane proteins; protonation; surface-enhanced infrared spectroscopy; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

