Mitochondrial dynamics in neuronal injury, development and plasticity
- PMID: 28154157
- PMCID: PMC5339882
- DOI: 10.1242/jcs.171017
Mitochondrial dynamics in neuronal injury, development and plasticity
Abstract
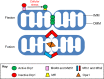
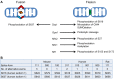
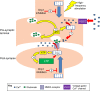
Mitochondria fulfill numerous cellular functions including ATP production, Ca2+ buffering, neurotransmitter synthesis and degradation, ROS production and sequestration, apoptosis and intermediate metabolism. Mitochondrial dynamics, a collective term for the processes of mitochondrial fission, fusion and transport, governs mitochondrial function and localization within the cell. Correct balance of mitochondrial dynamics is especially important in neurons as mutations in fission and fusion enzymes cause peripheral neuropathies and impaired development of the nervous system in humans. Regulation of mitochondrial dynamics is partly accomplished through post-translational modification of mitochondrial fission and fusion enzymes, in turn influencing mitochondrial bioenergetics and transport. The importance of post-translational regulation is highlighted by numerous neurodegenerative disorders associated with post-translational modification of the mitochondrial fission enzyme Drp1. Not surprisingly, mitochondrial dynamics also play an important physiological role in the development of the nervous system and synaptic plasticity. Here, we highlight recent findings underlying the mechanisms and regulation of mitochondrial dynamics in relation to neurological disease, as well as the development and plasticity of the nervous system.
Keywords: Bioenergetics; Dynamin-related protein 1; Mitochondrial fission; Mitochondrial fusion; Neurodegenerative disease; Synaptic plasticity.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Alexander C., Votruba M., Pesch U. E., Thiselton D. L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G. et al. (2000). OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 26, 211-215. 10.1038/79944 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

