Metabolic Alterations Contribute to Enhanced Inflammatory Cytokine Production in Irgm1-deficient Macrophages
- PMID: 28154172
- PMCID: PMC5377780
- DOI: 10.1074/jbc.M116.770735
Metabolic Alterations Contribute to Enhanced Inflammatory Cytokine Production in Irgm1-deficient Macrophages
Abstract
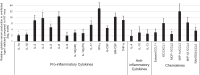
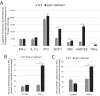
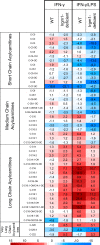
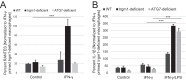
The immunity-related GTPases (IRGs) are a family of proteins that are induced by interferon (IFN)-γ and play pivotal roles in immune and inflammatory responses. IRGs ostensibly function as dynamin-like proteins that bind to intracellular membranes and promote remodeling and trafficking of those membranes. Prior studies have shown that loss of Irgm1 in mice leads to increased lethality to bacterial infections as well as enhanced inflammation to non-infectious stimuli; however, the mechanisms underlying these phenotypes are unclear. In the studies reported here, we found that uninfected Irgm1-deficient mice displayed high levels of serum cytokines typifying profound autoinflammation. Similar increases in cytokine production were also seen in cultured, IFN-γ-primed macrophages that lacked Irgm1. A series of metabolic studies indicated that the enhanced cytokine production was associated with marked metabolic changes in the Irgm1-deficient macrophages, including increased glycolysis and an accumulation of long chain acylcarnitines. Cells were exposed to the glycolytic inhibitor, 2-deoxyglucose, or fatty acid synthase inhibitors to perturb the metabolic alterations, which resulted in dampening of the excessive cytokine production. These results suggest that Irgm1 deficiency drives metabolic dysfunction in macrophages in a manner that is cell-autonomous and independent of infectious triggers. This may be a significant contributor to excessive inflammation seen in Irgm1-deficient mice in different contexts.
Keywords: Irgm1; cytokine induction; fatty acid metabolism; glycolysis; immunity-related GTPase; interferon gamma; macrophage; respiration.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Type I interferon signaling and peroxisomal dysfunction contribute to enhanced inflammatory cytokine production in IRGM1-deficient macrophages.J Biol Chem. 2024 Nov;300(11):107883. doi: 10.1016/j.jbc.2024.107883. Epub 2024 Oct 11. J Biol Chem. 2024. PMID: 39395806 Free PMC article.
-
Loss of the interferon-γ-inducible regulatory immunity-related GTPase (IRG), Irgm1, causes activation of effector IRG proteins on lysosomes, damaging lysosomal function and predicting the dramatic susceptibility of Irgm1-deficient mice to infection.BMC Biol. 2016 Apr 20;14:33. doi: 10.1186/s12915-016-0255-4. BMC Biol. 2016. PMID: 27098192 Free PMC article.
-
Balance of Irgm protein activities determines IFN-gamma-induced host defense.J Leukoc Biol. 2009 May;85(5):877-85. doi: 10.1189/jlb.1008599. Epub 2009 Jan 27. J Leukoc Biol. 2009. PMID: 19176402 Free PMC article.
-
Emerging themes in IFN-gamma-induced macrophage immunity by the p47 and p65 GTPase families.Immunobiology. 2007;212(9-10):771-84. doi: 10.1016/j.imbio.2007.09.018. Epub 2007 Nov 28. Immunobiology. 2007. PMID: 18086378 Free PMC article. Review.
-
Crucial role of interferon-gamma and stimulated macrophages in cardiovascular disease.Curr Vasc Pharmacol. 2006 Jul;4(3):205-13. doi: 10.2174/157016106777698379. Curr Vasc Pharmacol. 2006. PMID: 16842138 Review.
Cited by
-
Histone hyperacetylation mediates enhanced IL-1β production in LPS/IFN-γ-stimulated macrophages.Immunology. 2020 Jun;160(2):183-197. doi: 10.1111/imm.13183. Epub 2020 Apr 7. Immunology. 2020. PMID: 32061096 Free PMC article.
-
Irgm1 regulates metabolism and function in T cell subsets.Sci Rep. 2022 Jan 17;12(1):850. doi: 10.1038/s41598-021-04442-x. Sci Rep. 2022. PMID: 35039539 Free PMC article.
-
Loss of immunity-related GTPase GM4951 leads to nonalcoholic fatty liver disease without obesity.Nat Commun. 2022 Jul 16;13(1):4136. doi: 10.1038/s41467-022-31812-4. Nat Commun. 2022. PMID: 35842425 Free PMC article.
-
Partners in anti-crime: how interferon-inducible GTPases and autophagy proteins team up in cell-intrinsic host defense.Curr Opin Immunol. 2018 Oct;54:93-101. doi: 10.1016/j.coi.2018.06.008. Epub 2018 Jul 5. Curr Opin Immunol. 2018. PMID: 29986303 Free PMC article. Review.
-
Innate Immunity to Intracellular Pathogens: Balancing Microbial Elimination and Inflammation.Cell Host Microbe. 2017 Aug 9;22(2):166-175. doi: 10.1016/j.chom.2017.07.005. Cell Host Microbe. 2017. PMID: 28799902 Free PMC article. Review.
References
-
- Uthaiah R. C., Praefcke G. J., Howard J. C., and Herrmann C. (2003) IIGP1, an interferon-γ-inducible 47-kDa GTPase of the mouse, showing cooperative enzymatic activity and GTP-dependent multimerization. J. Biol. Chem. 278, 29336–29343 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

