IL-33-Mediated Expansion of Type 2 Innate Lymphoid Cells Protects from Progressive Glomerulosclerosis
- PMID: 28154198
- PMCID: PMC5491284
- DOI: 10.1681/ASN.2016080877
IL-33-Mediated Expansion of Type 2 Innate Lymphoid Cells Protects from Progressive Glomerulosclerosis
Abstract
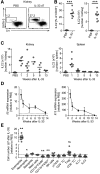
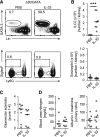
Innate lymphoid cells (ILCs) have an important role in the immune system's response to different forms of infectious and noninfectious pathologies. In particular, IL-5- and IL-13-producing type 2 ILCs (ILC2s) have been implicated in repair mechanisms that restore tissue integrity after injury. However, the presence of renal ILCs in humans has not been reported. In this study, we show that ILC populations are present in the healthy human kidney. A detailed characterization of kidney-residing ILC populations revealed that IL-33 receptor-positive ILC2s are a major ILC subtype in the kidney of humans and mice. Short-term IL-33 treatment in mice led to sustained expansion of IL-33 receptor-positive kidney ILC2s and ameliorated adriamycin-induced glomerulosclerosis. Furthermore, the expansion of ILC2s modulated the inflammatory response in the diseased kidney in favor of an anti-inflammatory milieu with a reduction of pathogenic myeloid cell infiltration and a marked accumulation of eosinophils that was required for tissue protection. In summary, kidney-residing ILC2s can be effectively expanded in the mouse kidney by IL-33 treatment and are central regulators of renal repair mechanisms. The presence of ILC2s in the human kidney tissue identifies these cells as attractive therapeutic targets for CKD in humans.
Keywords: IL-33; glomerulosclerosis; innate lymphoid cells.
Copyright © 2017 by the American Society of Nephrology.
Figures





Comment in
-
ILC2: There's a New Cell in Town.J Am Soc Nephrol. 2017 Jul;28(7):1953-1955. doi: 10.1681/ASN.2017040398. Epub 2017 May 31. J Am Soc Nephrol. 2017. PMID: 28566478 Free PMC article. No abstract available.
References
-
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 - PubMed
-
- Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 - PubMed
-
- Eberl G, Di Santo JP, Vivier E: The brave new world of innate lymphoid cells. Nat Immunol 16: 1–5, 2015 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

