Targeting iron metabolism in drug discovery and delivery
- PMID: 28154410
- PMCID: PMC5455971
- DOI: 10.1038/nrd.2016.248
Targeting iron metabolism in drug discovery and delivery
Abstract
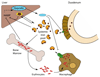
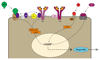
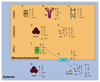
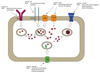
Iron fulfils a central role in many essential biochemical processes in human physiology; thus, proper processing of iron is crucial. Although iron metabolism is subject to relatively strict physiological control, numerous disorders, such as cancer and neurodegenerative diseases, have recently been linked to deregulated iron homeostasis. Consequently, iron metabolism constitutes a promising and largely unexploited therapeutic target for the development of new pharmacological treatments for these diseases. Several iron metabolism-targeted therapies are already under clinical evaluation for haematological disorders, and these and newly developed therapeutic agents are likely to have substantial benefit in the clinical management of iron metabolism-associated diseases, for which few efficacious treatments are currently available.
Conflict of interest statement
S.R. has restricted stocks in Merganser Biotech. S.R. is a consultant for Novartis Pharmaceuticals, Bayer Healthcare, Merganser Biotech and Keryx Pharmaceuticals. S.R. is a member of scientific advisory board of Merganser Biotech and Ionis Pharmaceuticals. T.L. is a member of scientific advisory board of Cristal Therapeutics B.V‥
Figures

References
-
- Rouault TA. Iron-sulfur proteins hiding in plain sight. Nat Chem Biol. 2015;11:442–445. - PubMed
-
- Hohenberger J, Ray K, Meyer K. The biology and chemistry of high-valent iron-oxo and iron-nitrido complexes. Nat Commun. 2012;3:720. - PubMed
-
- Ganz T. Systemic Iron Homeostasis. Physiol Rev. 2013;93:1721–1741. - PubMed
-
- Camaschella C. Iron-Deficiency Anemia. N Engl J Med. 2015;372:1832–1843. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

