Patient-Derived Xenograft Models of Non-Small Cell Lung Cancer and Their Potential Utility in Personalized Medicine
- PMID: 28154808
- PMCID: PMC5243815
- DOI: 10.3389/fonc.2017.00002
Patient-Derived Xenograft Models of Non-Small Cell Lung Cancer and Their Potential Utility in Personalized Medicine
Abstract
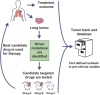
Traditional preclinical studies of cancer therapeutics have relied on the use of established human cell lines that have been adapted to grow in the laboratory and, therefore, may deviate from the cancer they were meant to represent. With the emphasis of cancer drug development shifting from non-specific cytotoxic agents to rationally designed molecularly targeted therapies or immunotherapy comes the need for better models with predictive value regarding therapeutic activity and response in clinical trials. Recently, the diversity and accessibility of immunodeficient mouse strains has greatly enhanced the production and utility of patient-derived xenograft (PDX) models for many tumor types, including non-small cell lung cancer (NSCLC). Combined with next-generation sequencing, NSCLC PDX mouse models offer an exciting tool for drug development and for studying targeted therapies while utilizing patient samples with the hope of eventually aiding in clinical decision-making. Here, we describe NSCLC PDX mouse models generated by us and others, their ability to reflect the parental tumors' histomorphological characteristics, as well as the effect of clonal selection and evolution on maintaining genomic integrity in low-passage PDXs compared to the donor tissue. We also raise vital questions regarding the practical utility of PDX and humanized PDX models in predicting patient response to therapy and make recommendations for addressing those questions. Once collaborations and standardized xenotransplantation and data management methods are established, NSCLC PDX mouse models have the potential to be universal and invaluable as a preclinical tool that guides clinical trials and standard therapeutic decisions.
Keywords: lung cancer; patient-derived xenograft; personalized medicine; precision medicine; preclinical trial.
Figures
References
-
- Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov (2014) 4:998–1013.10.1158/2159-8290.CD-14-0001 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

