Phloretin Attenuates Listeria monocytogenes Virulence Both In vitro and In vivo by Simultaneously Targeting Listeriolysin O and Sortase A
- PMID: 28154809
- PMCID: PMC5244253
- DOI: 10.3389/fcimb.2017.00009
Phloretin Attenuates Listeria monocytogenes Virulence Both In vitro and In vivo by Simultaneously Targeting Listeriolysin O and Sortase A
Abstract
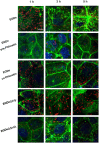
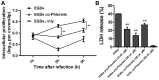
The critical roles of sortase A (SrtA) and listeriolysin O (LLO) in Listeria monocytogenes pathogenicity render these two virulence factors as ideal targets for the development of anti-virulence agents against L. monocytogenes infection. Additionally, the structures of SrtA and LLO are highly conserved among the members of sortase enzyme family and cholesterol dependent toxin family. Here, phloretin, a natural polyphenolic compound derived from apples and pears that has little anti-L. monocytogenes activity, was identified to simultaneously inhibit LLO expression and neutralize SrtA catalytic activity. Phloretin neutralized SrtA activity by causing a conformational change in the protein's active pocket, which prevented engagement with its substrate. Treatment with phloretin simultaneously reduced L. monocytogenes invasion into host cells and blocked the escape of vacuole-entrapped L. monocytogenes into cytoplasm. Further, L. monocytogenes-infected mice that received phloretin showed lower mortality, decreased bacterial burden and reduced pathological injury. Our results demonstrate that phloretin is a promising anti-infective therapeutic for infections caused by L. monocytogenes due to its simultaneous targeting of SrtA and LLO, which may result in fewer side effects than those caused by other antibiotics.
Keywords: Listeria monocytogenes; anti-infective therapy; listeriolysin O; phloretin; sortase A.
Figures
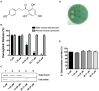
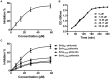
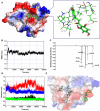
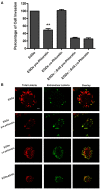

References
-
- Bandyopadhyay S., Valder C. R., Huynh H. G., Ren H., Allison W. S. (2002). The βG156C substitution in the F1-ATPase from the thermophilic Bacillus PS3 affects catalytic site cooperativity by destabilizing the closed conformation of the catalytic site. Biochemistry 41, 14421–14429. 10.1021/bi026243g - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

