Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: a multicenter randomized controlled trial
- PMID: 28155060
- PMCID: PMC5429256
- DOI: 10.1007/s10195-017-0442-2
Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: a multicenter randomized controlled trial
Abstract
Background: Infection is one of the main reasons for failure of orthopedic implants. Antibacterial coatings may prevent bacterial adhesion and biofilm formation, according to various preclinical studies. The aim of the present study is to report the first clinical trial on an antibiotic-loaded fast-resorbable hydrogel coating (Defensive Antibacterial Coating, DAC®) to prevent surgical site infection, in patients undergoing internal osteosynthesis for closed fractures.
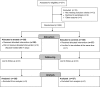
Materials and methods: In this multicenter randomized controlled prospective study, a total of 256 patients in five European orthopedic centers who were scheduled to receive osteosynthesis for a closed fracture, were randomly assigned to receive antibiotic-loaded DAC or to a control group (without coating). Pre- and postoperative assessment of laboratory tests, wound healing, clinical scores and X-rays were performed at fixed time intervals.
Results: Overall, 253 patients were available with a mean follow-up of 18.1 ± 4.5 months (range 12-30). On average, wound healing, clinical scores, laboratory tests and radiographic findings did not show any significant difference between the two groups. Six surgical site infections (4.6%) were observed in the control group compared to none in the treated group (P < 0.03). No local or systemic side-effects related to the DAC hydrogel product were observed and no detectable interference with bone healing was noted.
Conclusions: The use of a fast-resorbable antibiotic-loaded hydrogel implant coating provides a reduced rate of post-surgical site infections after internal osteosynthesis for closed fractures, without any detectable adverse event or side-effects.
Level of evidence: 2.
Keywords: Coating; DAC; Hydrogel; Infection; Osteosynthesis; Prevention.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Patient consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All patients gave the informed consent prior being included into the study. All procedures involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments. The study was approved by the responsible Research Ethics Committee or Institutional Review Board.
Funding
The study was performed within the 7th European Framework Programme (project #277988) and funded by the European Commission and the participating partners (clinical institutions and the following private companies: Novagenit SRL, Mezzolombardo, Italy, acting as project leader; AdlerOrtho SRL, Bologna, Italy; Arcos SARL, Brignoles, France; Belgafix SPRL, Drogenbos, Belgium).
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials