Prenatal thalamic waves regulate cortical area size prior to sensory processing
- PMID: 28155854
- PMCID: PMC5296753
- DOI: 10.1038/ncomms14172
Prenatal thalamic waves regulate cortical area size prior to sensory processing
Abstract
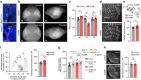
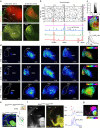
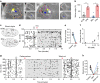
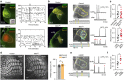
The cerebral cortex is organized into specialized sensory areas, whose initial territory is determined by intracortical molecular determinants. Yet, sensory cortical area size appears to be fine tuned during development to respond to functional adaptations. Here we demonstrate the existence of a prenatal sub-cortical mechanism that regulates the cortical areas size in mice. This mechanism is mediated by spontaneous thalamic calcium waves that propagate among sensory-modality thalamic nuclei up to the cortex and that provide a means of communication among sensory systems. Wave pattern alterations in one nucleus lead to changes in the pattern of the remaining ones, triggering changes in thalamic gene expression and cortical area size. Thus, silencing calcium waves in the auditory thalamus induces Rorβ upregulation in a neighbouring somatosensory nucleus preluding the enlargement of the barrel-field. These findings reveal that embryonic thalamic calcium waves coordinate cortical sensory area patterning and plasticity prior to sensory information processing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Miyashita-Lin E., Hevner R., Wassarman K., Martinez S. & Rubenstein J. Early neocortical regionalization in the absence of thalamic innervation. Science 285, 906–909 (1999). - PubMed
-
- O'Leary D. D., Chou S. J. & Sahara S. Area patterning of the mammalian cortex. Neuron 56, 252–269 (2007). - PubMed
-
- Grove E. A. & Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu. Rev. Neurosci. 26, 355–380 (2003). - PubMed
-
- Garel S., Huffman K. J. & Rubenstein J. L. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development 130, 1903–1914 (2003). - PubMed
-
- Rakic P. Specification of cerebral cortical areas. Science 241, 170–176 (1988). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

