Successful post-exposure prophylaxis of Ebola infected non-human primates using Ebola glycoprotein-specific equine IgG
- PMID: 28155869
- PMCID: PMC5290740
- DOI: 10.1038/srep41537
Successful post-exposure prophylaxis of Ebola infected non-human primates using Ebola glycoprotein-specific equine IgG
Abstract
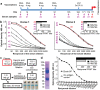
Herein we describe production of purified equine IgG obtained from horses immunized with plasmid DNA followed by boosting with Kunjin replicon virus-like particles both encoding a modified Ebola glycoprotein. Administration of the equine IgG over 5 days to cynomolgus macaques infected 24 hours previously with a lethal dose of Ebola virus suppressed viral loads by more than 5 logs and protected animals from mortality. Animals generated their own Ebola glycoprotein-specific IgG responses 9-15 days after infection, with circulating virus undetectable by day 15-17. Such equine IgG may find utility as a post-exposure prophylactic for Ebola infection and provides a low cost, scalable alternative to monoclonal antibodies, with extensive human safety data and WHO-standardized international manufacturing capability available in both high and low income countries.
Conflict of interest statement
No competing financial interests for all authors. JFF is employed by the United Nations and views expressed herein are those of the author(s) and do not necessarily reflect the views of the United Nations.
Figures

References
-
- Cardile A. P., Downey L. G., Wiseman P. D., Warren T. K. & Bavari S. Antiviral therapeutics for the treatment of Ebola virus infection. Curr Opin Pharmacol 30, 138–143 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

