New perspectives on corpora amylacea in the human brain
- PMID: 28155917
- PMCID: PMC5290524
- DOI: 10.1038/srep41807
New perspectives on corpora amylacea in the human brain
Abstract
Corpora amylacea are structures of unknown origin and function that appear with age in human brains and are profuse in selected brain areas in several neurodegenerative conditions. They are constituted of glucose polymers and may contain waste elements derived from different cell types. As we previously found on particular polyglucosan bodies in mouse brain, we report here that corpora amylacea present some neo-epitopes that can be recognized by natural antibodies, a certain kind of antibodies that are involved in tissue homeostasis. We hypothesize that corpora amylacea, and probably some other polyglucosan bodies, are waste containers in which deleterious or residual products are isolated to be later eliminated through the action of the innate immune system. In any case, the presence of neo-epitopes on these structures and the existence of natural antibodies directed against them could become a new focal point for the study of both age-related and degenerative brain processes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



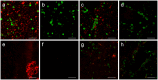
References
-
- Cavanagh J. B. Corpora-amylacea and the family of polyglucosan diseases. Brain Res. Rev. 29, 265–295 (1999). - PubMed
-
- Pirici D. & Margaritescu C. Corpora amylacea in aging brain and age-related brain disorders. J. Aging Gerontol. 2, 33–57 (2014).
-
- Ferraro A. & Damon L. A. The histogenesis of amyloid bodies in the central nervous system. Arch. Pathol. 12, 229–244 (1931).
-
- Sakai M., Austin J., Witmer F. & Trueb L. Studies of corpora amylacea: Isolation and preliminary characterization by chemical and histochemical techniques. Arch. Neurol. 21, 526–544 (1969). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

