BACE1 Dynamics Upon Inhibition with a BACE Inhibitor and Correlation to Downstream Alzheimer's Disease Markers in Elderly Healthy Participants
- PMID: 28157093
- PMCID: PMC5325057
- DOI: 10.3233/JAD-160829
BACE1 Dynamics Upon Inhibition with a BACE Inhibitor and Correlation to Downstream Alzheimer's Disease Markers in Elderly Healthy Participants
Abstract
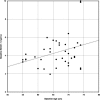
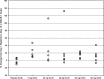
The β-site amyloid-β protein precursor (AβPP) cleaving enzyme-1 (BACE1) is the rate limiting enzyme in the generation of amyloid-β peptide (Aβ) from AβPP, one of the major pathways in Alzheimer's disease (AD) pathology. Increased BACE1 levels and activity have been reported in the brain of patients with sporadic AD. Therefore, changes of BACE1 levels in the cerebrospinal fluid (CSF) have also been investigated as a possible biomarker of the disease. We analyzed BACE1 levels in CSF of elderly healthy participants before and after chronic treatment with a BACE inhibitor (BACEi) and evaluated the correlation between BACE1 levels and downstream AD markers. Overall, BACE1 CSF levels showed strong correlations to all downstream AD markers investigated. This is the first reported finding that shows BACE1 levels in CSF were well correlated to its end product Aβ1 - 42. As previously described, BACE1 levels were strongly correlated to total-tau and phosphorylated tau levels in CSF. Generally, chronic BACE inhibition did not influence BACE1 CSF protein levels. Follow-up studies including early-stage AD pathophysiology and prodromal AD patients will help to understand the importance of measuring BACE1 routinely in daily clinical practice and AD clinical trials.
Keywords: AD markers; Alzheimer’s disease; BACE-1; JNJ-54861911; β-secretase enzyme.
Figures



Similar articles
-
Pharmacodynamics of atabecestat (JNJ-54861911), an oral BACE1 inhibitor in patients with early Alzheimer's disease: randomized, double-blind, placebo-controlled study.Alzheimers Res Ther. 2018 Aug 23;10(1):85. doi: 10.1186/s13195-018-0415-6. Alzheimers Res Ther. 2018. PMID: 30134967 Free PMC article. Clinical Trial.
-
Soluble BACE-1 Activity and sAβPPβ Concentrations in Alzheimer's Disease and Age-Matched Healthy Control Cerebrospinal Fluid from the Alzheimer's Disease Neuroimaging Initiative-1 Baseline Cohort.J Alzheimers Dis. 2015;46(2):431-40. doi: 10.3233/JAD-142778. J Alzheimers Dis. 2015. PMID: 25790831 Free PMC article.
-
Prevention of tau increase in cerebrospinal fluid of APP transgenic mice suggests downstream effect of BACE1 inhibition.Alzheimers Dement. 2017 Jun;13(6):701-709. doi: 10.1016/j.jalz.2016.09.005. Epub 2016 Oct 14. Alzheimers Dement. 2017. PMID: 27750032
-
Investigational BACE inhibitors for the treatment of Alzheimer's disease.Expert Opin Investig Drugs. 2019 Nov;28(11):967-975. doi: 10.1080/13543784.2019.1683160. Epub 2019 Oct 29. Expert Opin Investig Drugs. 2019. PMID: 31661331 Review.
-
Beta-secretase (BACE) as a drug target for Alzheimer's disease.Adv Drug Deliv Rev. 2002 Dec 7;54(12):1589-602. doi: 10.1016/s0169-409x(02)00157-6. Adv Drug Deliv Rev. 2002. PMID: 12453676 Review.
Cited by
-
Bridging the Gap Between Fluid Biomarkers for Alzheimer's Disease, Model Systems, and Patients.Front Aging Neurosci. 2020 Sep 2;12:272. doi: 10.3389/fnagi.2020.00272. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32982716 Free PMC article. Review.
-
BACE1-Dependent Neuregulin-1 Signaling: An Implication for Schizophrenia.Front Mol Neurosci. 2017 Sep 25;10:302. doi: 10.3389/fnmol.2017.00302. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28993723 Free PMC article. Review.
-
Lessons Learned from Alzheimer Disease: Clinical Trials with Negative Outcomes.Clin Transl Sci. 2018 Mar;11(2):147-152. doi: 10.1111/cts.12491. Epub 2017 Aug 2. Clin Transl Sci. 2018. PMID: 28767185 Free PMC article. Review. No abstract available.
-
Characterization of Cerebrospinal Fluid BACE1 Species.Mol Neurobiol. 2019 Dec;56(12):8603-8616. doi: 10.1007/s12035-019-01677-8. Epub 2019 Jul 9. Mol Neurobiol. 2019. PMID: 31290061
-
Amyloid-β1-43 cerebrospinal fluid levels and the interpretation of APP, PSEN1 and PSEN2 mutations.Alzheimers Res Ther. 2020 Sep 11;12(1):108. doi: 10.1186/s13195-020-00676-5. Alzheimers Res Ther. 2020. PMID: 32917274 Free PMC article.
References
-
- Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ (2013) Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216. - PMC - PubMed
-
- Strozyk D, Blennow K, White LR, Launer LJ (2003) CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60, 652–656. - PubMed
-
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC (2006) [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology 67, 446–452. - PubMed
-
- Buerger K, Ewers M, Pirttila T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H (2006) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 129, 3035–3041. - PubMed
-
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

