Enhancing the Biological Relevance of Machine Learning Classifiers for Reverse Vaccinology
- PMID: 28157153
- PMCID: PMC5343848
- DOI: 10.3390/ijms18020312
Enhancing the Biological Relevance of Machine Learning Classifiers for Reverse Vaccinology
Abstract
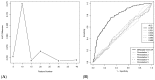
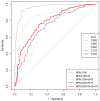
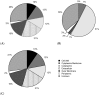
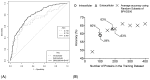
Reverse vaccinology (RV) is a bioinformatics approach that can predict antigens with protective potential from the protein coding genomes of bacterial pathogens for subunit vaccine design. RV has become firmly established following the development of the BEXSERO® vaccine against Neisseria meningitidis serogroup B. RV studies have begun to incorporate machine learning (ML) techniques to distinguish bacterial protective antigens (BPAs) from non-BPAs. This research contributes significantly to the RV field by using permutation analysis to demonstrate that a signal for protective antigens can be curated from published data. Furthermore, the effects of the following on an ML approach to RV were also assessed: nested cross-validation, balancing selection of non-BPAs for subcellular localization, increasing the training data, and incorporating greater numbers of protein annotation tools for feature generation. These enhancements yielded a support vector machine (SVM) classifier that could discriminate BPAs (n = 200) from non-BPAs (n = 200) with an area under the curve (AUC) of 0.787. In addition, hierarchical clustering of BPAs revealed that intracellular BPAs clustered separately from extracellular BPAs. However, no immediate benefit was derived when training SVM classifiers on data sets exclusively containing intra- or extracellular BPAs. In conclusion, this work demonstrates that ML classifiers have great utility in RV approaches and will lead to new subunit vaccines in the future.
Keywords: bacterial pathogen; bacterial protective antigen; machine learning; reverse vaccinology; support vector machine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pizza M., Scarlato V., Masignani M.M., Giuliani B., Arico M., Comanducci G.T., Jennings L., Baldi E., Bartolini B., Capecchi B., et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. - DOI - PubMed
-
- Yu N.Y., Wagner J.R., Laird M.R., Melli G., Rey S., Lo R., Dao P., Sahinalp S.C., Ester M., Foster L.J. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–1615. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

