Autophagy induced by DAMPs facilitates the inflammation response in lungs undergoing ischemia-reperfusion injury through promoting TRAF6 ubiquitination
- PMID: 28157209
- PMCID: PMC5384028
- DOI: 10.1038/cdd.2017.1
Autophagy induced by DAMPs facilitates the inflammation response in lungs undergoing ischemia-reperfusion injury through promoting TRAF6 ubiquitination
Abstract
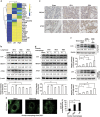
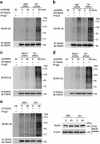
Lung ischemia-reperfusion (I/R) injury remains one of the most common complications after various cardiopulmonary surgeries. The inflammation response triggered by the released damage-associated molecular patterns (DAMPs) aggravates lung tissue damage. However, little is known about the role of autophagy in the pathogenesis of lung I/R injury. Here, we report that a variety of inflammation-related and autophagy-associated genes are rapidly upregulated, which facilitate the inflammation response in a minipig lung I/R injury model. Left lung I/R injury triggered inflammatory cytokine production and activated the autophagy flux as evidenced in crude lung tissues and alveolar macrophages. This was associated with the release of DAMPs, such as high mobility group protein B1 (HMGB1) and heat shock protein 60 (HSP60). Indeed, treatment with recombinant HMGB1 or HSP60 induced autophagy in alveolar macrophages, whereas autophagy inhibition by knockdown of ATG7 or BECN1 markedly reduced DAMP-triggered production of inflammatory cytokines including IL-1β, TNF and IL12 in alveolar macrophages. This appeared to be because of decreased activation of MAPK and NF-κB signaling. Furthermore, knockdown of ATG7 or BECN1 inhibited Lys63 (K63)-linked ubiquitination of TNF receptor-associated factor 6 (TRAF6) in DAMP-treated alveolar macrophages. Consistently, treatment with 3-MA inhibited K63-linked ubiquitination of TRAF6 in I/R-injured lung tissues in vivo. Collectively, these results indicate that autophagy triggered by DAMPs during lung I/R injury amplifies the inflammatory response through enhancing K63-linked ubiquitination of TRAF6 and activation of the downstream MAPK and NF-κB signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Artesunate Attenuates Pro-Inflammatory Cytokine Release from Macrophages by Inhibiting TLR4-Mediated Autophagic Activation via the TRAF6-Beclin1-PI3KC3 Pathway.Cell Physiol Biochem. 2018;47(2):475-488. doi: 10.1159/000489982. Epub 2018 May 22. Cell Physiol Biochem. 2018. PMID: 29794440
-
NLRC3 alleviates hypoxia/reoxygenation induced inflammation in RAW264.7 cells by inhibiting K63-linked ubiquitination of TRAF6.Hepatobiliary Pancreat Dis Int. 2020 Oct;19(5):455-460. doi: 10.1016/j.hbpd.2020.04.003. Epub 2020 May 3. Hepatobiliary Pancreat Dis Int. 2020. PMID: 32386989
-
TLR4-HMGB1-, MyD88- and TRIF-dependent signaling in mouse intestinal ischemia/reperfusion injury.World J Gastroenterol. 2015 Jul 21;21(27):8314-25. doi: 10.3748/wjg.v21.i27.8314. World J Gastroenterol. 2015. PMID: 26217083 Free PMC article.
-
Decoding cell death signals in liver inflammation.J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
-
Advances in lung ischemia/reperfusion injury: unraveling the role of innate immunity.Inflamm Res. 2024 Mar;73(3):393-405. doi: 10.1007/s00011-023-01844-7. Epub 2024 Jan 24. Inflamm Res. 2024. PMID: 38265687 Review.
Cited by
-
TRAF6 triggers Mycobacterium-infected host autophagy through Rab7 ubiquitination.Cell Death Discov. 2023 Nov 28;9(1):427. doi: 10.1038/s41420-023-01731-4. Cell Death Discov. 2023. PMID: 38016969 Free PMC article.
-
Classic and Current Opinions in Human Organ and Tissue Transplantation.Cureus. 2022 Nov 1;14(11):e30982. doi: 10.7759/cureus.30982. eCollection 2022 Nov. Cureus. 2022. PMID: 36337306 Free PMC article. Review.
-
The role of the LncRNA-FA2H-2-MLKL pathway in atherosclerosis by regulation of autophagy flux and inflammation through mTOR-dependent signaling.Cell Death Differ. 2019 Sep;26(9):1670-1687. doi: 10.1038/s41418-018-0235-z. Epub 2019 Jan 25. Cell Death Differ. 2019. PMID: 30683918 Free PMC article.
-
Autophagy, Unfolded Protein Response, and Neuropilin-1 Cross-Talk in SARS-CoV-2 Infection: What Can Be Learned from Other Coronaviruses.Int J Mol Sci. 2021 Jun 1;22(11):5992. doi: 10.3390/ijms22115992. Int J Mol Sci. 2021. PMID: 34206057 Free PMC article. Review.
-
Therapeutic targets and interventional strategies in COVID-19: mechanisms and clinical studies.Signal Transduct Target Ther. 2021 Aug 26;6(1):317. doi: 10.1038/s41392-021-00733-x. Signal Transduct Target Ther. 2021. PMID: 34446699 Free PMC article. Review.
References
-
- van Golen RF, Reiniers MJ, Olthof PB, van Gulik TM, Heger M. Sterile inflammation in hepatic ischemia/reperfusion injury: present concepts and potential therapeutics. J Gastroenterol Hepatol 2013; 28: 394–400. - PubMed
-
- den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol 2010; 299: H1283–H1299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

