WNT/β-Catenin signaling pathway regulates non-tumorigenesis of human embryonic stem cells co-cultured with human umbilical cord mesenchymal stem cells
- PMID: 28157212
- PMCID: PMC5291217
- DOI: 10.1038/srep41913
WNT/β-Catenin signaling pathway regulates non-tumorigenesis of human embryonic stem cells co-cultured with human umbilical cord mesenchymal stem cells
Abstract
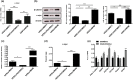
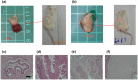
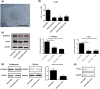
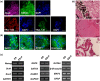
Human pluripotent stem cells harbor hope in regenerative medicine, but have limited application in treating clinical diseases due to teratoma formation. Our previous study has indicated that human umbilical cord mesenchymal stem cells (HUCMSC) can be adopted as non-teratogenenic feeders for human embryonic stem cells (hESC). This work describes the mechanism of non-tumorigenesis of that feeder system. In contrast with the mouse embryonic fibroblast (MEF) feeder, HUCMSC down-regulates the WNT/β-catenin/c-myc signaling in hESC. Thus, adding β-catenin antagonist (FH535 or DKK1) down-regulates β-catenin and c-myc expressions, and suppresses tumorigenesis (3/14 vs. 4/4, p = 0.01) in hESC fed with MEF, while adding the β-catenin enhancer (LiCl or 6-bromoindirubin-3'-oxime) up-regulates the expressions, and has a trend (p = 0.056) to promote tumorigenesis (2/7 vs. 0/21) in hESC fed with HUCMSC. Furthermore, FH535 supplement does not alter the pluripotency of hESC when fed with MEF, as indicated by the differentiation capabilities of the three germ layers. Taken together, this investigation concludes that WNT/β-catenin/c-myc pathway causes the tumorigenesis of hESC on MEF feeder, and β-catenin antagonist may be adopted as a tumor suppressor.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Human umbilical cord mesenchymal stem cells support nontumorigenic expansion of human embryonic stem cells.Cell Transplant. 2012;21(7):1515-27. doi: 10.3727/096368912X647199. Cell Transplant. 2012. PMID: 22732188
-
Development of a Xeno-Free Feeder-Layer System from Human Umbilical Cord Mesenchymal Stem Cells for Prolonged Expansion of Human Induced Pluripotent Stem Cells in Culture.PLoS One. 2016 Feb 16;11(2):e0149023. doi: 10.1371/journal.pone.0149023. eCollection 2016. PLoS One. 2016. PMID: 26882313 Free PMC article.
-
Dickkopf Wnt signaling pathway inhibitor 1 regulates the differentiation of mouse embryonic stem cells in vitro and in vivo.Mol Med Rep. 2016 Jan;13(1):720-30. doi: 10.3892/mmr.2015.4586. Epub 2015 Nov 19. Mol Med Rep. 2016. PMID: 26648540 Free PMC article.
-
Therapeutic potential of umbilical cord mesenchymal stem cells with Wnt/β-catenin signaling pathway pre-activated for the treatment of diabetic wounds.Eur Rev Med Pharmacol Sci. 2014;18(17):2460-4. Eur Rev Med Pharmacol Sci. 2014. PMID: 25268090 Review.
-
Wnt/β-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming.Stem Cell Rev Rep. 2011 Nov;7(4):836-46. doi: 10.1007/s12015-011-9275-1. Stem Cell Rev Rep. 2011. PMID: 21603945 Review.
Cited by
-
PAK6 promotes cervical cancer progression through activation of the Wnt/β-catenin signaling pathway.Oncol Lett. 2020 Sep;20(3):2387-2395. doi: 10.3892/ol.2020.11797. Epub 2020 Jul 1. Oncol Lett. 2020. PMID: 32782556 Free PMC article.
-
Human fallopian tube epithelial cells exhibit stemness features, self-renewal capacity, and Wnt-related organoid formation.J Biomed Sci. 2020 Feb 8;27(1):32. doi: 10.1186/s12929-019-0602-1. J Biomed Sci. 2020. PMID: 32035490 Free PMC article.
-
Essentiality of CTNNB1 in Malignant Transformation of Human Embryonic Stem Cells under Long-Term Suboptimal Conditions.Stem Cells Int. 2020 Sep 24;2020:5823676. doi: 10.1155/2020/5823676. eCollection 2020. Stem Cells Int. 2020. PMID: 33029148 Free PMC article.
-
Tumorigenicity-associated characteristics of human iPS cell lines.PLoS One. 2018 Oct 4;13(10):e0205022. doi: 10.1371/journal.pone.0205022. eCollection 2018. PLoS One. 2018. PMID: 30286143 Free PMC article.
-
Estradiol and Progesterone Induced Differentiation and Increased Stemness Gene Expression of Human Fallopian Tube Epithelial Cells.J Cancer. 2019 Jun 2;10(13):3028-3036. doi: 10.7150/jca.30588. eCollection 2019. J Cancer. 2019. PMID: 31281480 Free PMC article.
References
-
- Thomson J. A. et al.. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

