The citrus flavanone naringenin impairs dengue virus replication in human cells
- PMID: 28157234
- PMCID: PMC5291091
- DOI: 10.1038/srep41864
The citrus flavanone naringenin impairs dengue virus replication in human cells
Erratum in
-
Corrigendum: The citrus flavanone naringenin impairs dengue virus replication in human cells.Sci Rep. 2017 Mar 14;7:43976. doi: 10.1038/srep43976. Sci Rep. 2017. PMID: 28290539 Free PMC article. No abstract available.
Abstract
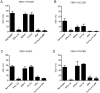
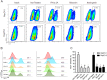
Dengue is one of the most significant health problems in tropical and sub-tropical regions throughout the world. Nearly 390 million cases are reported each year. Although a vaccine was recently approved in certain countries, an anti-dengue virus drug is still needed. Fruits and vegetables may be sources of compounds with medicinal properties, such as flavonoids. This study demonstrates the anti-dengue virus activity of the citrus flavanone naringenin, a class of flavonoid. Naringenin prevented infection with four dengue virus serotypes in Huh7.5 cells. Additionally, experiments employing subgenomic RepDV-1 and RepDV-3 replicon systems confirmed the ability of naringenin to inhibit dengue virus replication. Antiviral activity was observed even when naringenin was used to treat Huh7.5 cells 24 h after dengue virus exposure. Finally, naringenin anti-dengue virus activity was demonstrated in primary human monocytes infected with dengue virus sertoype-4, supporting the potential use of naringenin to control dengue virus replication. In conclusion, naringenin is a suitable candidate molecule for the development of specific dengue virus treatments.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- WHO. Dengue: guidelines for diagnosis, treatment, prevention, and control. Spec. Program. Res. Train. Trop. Dis. 147, doi: WHO/HTM/NTD/DEN/2009.1 (2009).
-
- Chambers T. J., Hahn C. S., Galler R. & Rice C. M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44, 649–688 (1990). - PubMed
-
- Hadinegoro S. R. et al.. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 373, 1195–1206 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

