Myocardial fibrosis: biomedical research from bench to bedside
- PMID: 28157267
- PMCID: PMC5299507
- DOI: 10.1002/ejhf.696
Myocardial fibrosis: biomedical research from bench to bedside
Abstract
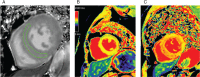
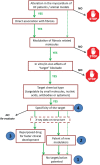
Myocardial fibrosis refers to a variety of quantitative and qualitative changes in the interstitial myocardial collagen network that occur in response to cardiac ischaemic insults, systemic diseases, drugs, or any other harmful stimulus affecting the circulatory system or the heart itself. Myocardial fibrosis alters the architecture of the myocardium, facilitating the development of cardiac dysfunction, also inducing arrhythmias, influencing the clinical course and outcome of heart failure patients. Focusing on myocardial fibrosis may potentially improve patient care through the targeted diagnosis and treatment of emerging fibrotic pathways. The European Commission funded the FIBROTARGETS consortium as a multinational academic and industrial consortium with the primary aim of performing a systematic and collaborative search of targets of myocardial fibrosis, and then translating these mechanisms into individualized diagnostic tools and specific therapeutic pharmacological options for heart failure. This review focuses on those methodological and technological aspects considered and developed by the consortium to facilitate the transfer of the new mechanistic knowledge on myocardial fibrosis into potential biomedical applications.
Keywords: Animal models; Biomarkers; Cardiac imaging; Myocardial fibrosis.
© 2017 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures



References
-
- Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast‐mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 2013;10:15–26. - PubMed
-
- Jellis C, Martin J, Narula J, Marwick TH. Assessment of nonischemic myocardial fibrosis. J Am Coll Cardiol 2010;56:89–97. - PubMed
-
- Li AH, Liu PP, Villarreal FJ, Garcia RA. Dynamic changes in myocardial matrix and relevance to disease: translational perspectives. Circ Res 2014;114:916–927. - PubMed
-
- Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J Mol Cell Cardiol 1989;21:121–131. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

