Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling
- PMID: 28157701
- PMCID: PMC5400538
- DOI: 10.18632/oncotarget.14982
Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling
Abstract
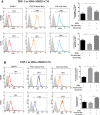
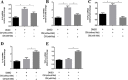
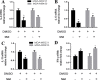
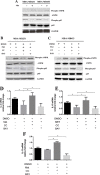
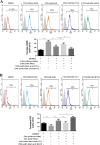
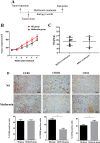
Accumulating evidence is indicating metformin to possess the potential ability in preventing tumor development and suppressing cancer growth. However, the exact mechanism of its antitumorigenic effects is still not clear. We found that metformin suppressed the ability of cancer to skew macrophage toward M2 phenotype. Metformin treated cancer cells increased macrophage expression of M1-related cytokines IL-12 and TNF-α and attenuated M2-related cytokines IL-8, IL-10, and TGF-β expression. Furthermore, metformin treated cancer cells displayed inhibited secretion of IL-4, IL-10 and IL-13; cytokines important for inducing M2 macrophages. Conversely, M1 inducing cytokine IFN-γ was upper-regulated in cancer cells. Additionally, through increasing AMPK and p65 phosphorylation, metformin treatment activated AMPK-NF-κB signaling of cancer cells that participate in regulating M1 and M2 inducing cytokines expression. Moreover, Compound C, an AMPK inhibitor, significantly increased IL-4, IL-10, and IL-13 expression while BAY-117082, an NF-κB inhibitor, decreased expression. In metformin-treated tumor tissue, the percentage of M2-like macrophages decreased while M1-like macrophages increased. These findings suggest that metformin activates cancer AMPK-NF-κB signaling, a pathway involved in regulating M1/M2 expression and inducing genes for macrophage polarization to anti-tumor phenotype.
Keywords: NF-κB; breast cancer; macrophage polarization; metformin; microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Chowdhury TA. Diabetes and cancer. QJM: monthly journal of the Association of Physicians. 2010;103:905–915. - PubMed
-
- Mukhtar RA, Nseyo O, Campbell MJ, Esserman LJ. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev Mol Diagn. 2011;11:91–100. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

