The interference of Notch1 target Hes1 affects cell growth, differentiation and invasiveness of glioblastoma stem cells through modulation of multiple oncogenic targets
- PMID: 28157712
- PMCID: PMC5392293
- DOI: 10.18632/oncotarget.15013
The interference of Notch1 target Hes1 affects cell growth, differentiation and invasiveness of glioblastoma stem cells through modulation of multiple oncogenic targets
Abstract
The invasive and lethal nature of Glioblastoma multiforme (GBM) necessitates the continuous identification of molecular targets and search of efficacious therapies to inhibit GBM growth. The GBM resistance to chemotherapy and radiation it is attributed to the existence of a rare fraction of cancer stem cells (CSC) that we have identified within the tumor core and in peritumor tissue of GBM. Since Notch1 pathway is a potential therapeutic target in brain cancer, earlier we highlighted that pharmacological inhibition of Notch1 signalling by γ-secretase inhibitor-X (GSI-X), reduced cell growth of some c-CSC than to their respective p-CSC, but produced negligible effects on cell cycle distribution, apoptosis and cell invasion. In the current study, we assessed the effects of Hes1-targeted shRNA, a Notch1 gene target, specifically on GBM CSC refractory to GSI-X. Depletion of Hes1 protein induces major changes in cell morphology, cell growth rate and in the invasive ability of shHes1-CSC in response to growth factor EGF. shHes1-CSC show a decrease of the stemness marker Nestin concurrently to a marked increase of neuronal marker MAP2 compared to pLKO.1-CSC. Those effects correlated with repression of EGFR protein and modulation of Stat3 phosphorylation at Y705 and S727 residues. In the last decade Stat3 has gained attention as therapeutic target in cancer but there is not yet any approved Stat3-based glioma therapy. Herein, we report that exposure to a Stat3/5 inhibitor, induced apoptosis either in shHes1-CSC or control cells. Taken together, Hes1 seems to be a favorable target but not sufficient itself to target GBM efficaciously, therefore a possible pharmacological intervention should provide for the use of anti-Stat3/5 drugs either alone or in combination regimen.
Keywords: Hes1; Notch1; differentiation; glioblastoma stem cells; self-renewal.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

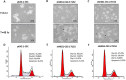
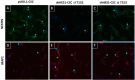
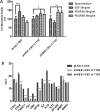

Similar articles
-
PDGF receptor alpha inhibition induces apoptosis in glioblastoma cancer stem cells refractory to anti-Notch and anti-EGFR treatment.Mol Cancer. 2014 Nov 8;13:247. doi: 10.1186/1476-4598-13-247. Mol Cancer. 2014. PMID: 25380967 Free PMC article.
-
PDGFRα depletion attenuates glioblastoma stem cells features by modulation of STAT3, RB1 and multiple oncogenic signals.Oncotarget. 2016 Aug 16;7(33):53047-53063. doi: 10.18632/oncotarget.10132. Oncotarget. 2016. PMID: 27344175 Free PMC article.
-
Effect of the STAT3 inhibitor STX-0119 on the proliferation of cancer stem-like cells derived from recurrent glioblastoma.Int J Oncol. 2013 Jul;43(1):219-27. doi: 10.3892/ijo.2013.1916. Epub 2013 Apr 23. Int J Oncol. 2013. PMID: 23612755
-
STAT3 as a Therapeutic Target for Glioblastoma.Anticancer Agents Med Chem. 2010 Sep;10(7):512-9. doi: 10.2174/187152010793498636. Anticancer Agents Med Chem. 2010. PMID: 20879983 Review.
-
The role of STAT3 in glioblastoma progression through dual influences on tumor cells and the immune microenvironment.Mol Cell Endocrinol. 2017 Aug 15;451:53-65. doi: 10.1016/j.mce.2017.01.004. Epub 2017 Jan 12. Mol Cell Endocrinol. 2017. PMID: 28089821 Review.
Cited by
-
Eukaryotic initiation factor 4 A-3 promotes glioblastoma growth and invasion through the Notch1-dependent pathway.BMC Cancer. 2023 Jun 15;23(1):550. doi: 10.1186/s12885-023-10946-8. BMC Cancer. 2023. PMID: 37322413 Free PMC article.
-
Autophagy suppresses self-renewal ability and tumorigenicity of glioma-initiating cells and promotes Notch1 degradation.Cell Death Dis. 2018 Oct 18;9(11):1063. doi: 10.1038/s41419-018-0957-3. Cell Death Dis. 2018. PMID: 30337536 Free PMC article.
-
Glioblastoma Stem Cell-Derived Exosomes Enhance Stemness and Tumorigenicity of Glioma Cells by Transferring Notch1 Protein.Cell Mol Neurobiol. 2020 Jul;40(5):767-784. doi: 10.1007/s10571-019-00771-8. Epub 2019 Dec 18. Cell Mol Neurobiol. 2020. PMID: 31853695 Free PMC article.
-
CircAPLP2 regulates the proliferation and metastasis of colorectal cancer by targeting miR-101-3p to activate the Notch signalling pathway.Am J Transl Res. 2020 Jun 15;12(6):2554-2569. eCollection 2020. Am J Transl Res. 2020. PMID: 32655790 Free PMC article.
-
Notch1 signaling pathway promotes invasion, self-renewal and growth of glioma initiating cells via modulating chemokine system CXCL12/CXCR4.J Exp Clin Cancer Res. 2019 Aug 5;38(1):339. doi: 10.1186/s13046-019-1319-4. J Exp Clin Cancer Res. 2019. PMID: 31382985 Free PMC article.
References
-
- Fidoamore A, Cristiano L, Antonosante A, d’Angelo M, Di Giacomo E, Astarita C, Giordano A, Ippoliti R, Benedetti E, Cimini A. Glioblastoma Stem Cells Microenvironment: The Paracrine Roles of the Niche in Drug and Radioresistance. Stem Cells Int. 2016;2016:6809105. doi: 10.1155/2016/6809105. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

