RB inactivation in keratin 18 positive thymic epithelial cells promotes non-cell autonomous T cell hyperproliferation in genetically engineered mice
- PMID: 28158249
- PMCID: PMC5291521
- DOI: 10.1371/journal.pone.0171510
RB inactivation in keratin 18 positive thymic epithelial cells promotes non-cell autonomous T cell hyperproliferation in genetically engineered mice
Abstract
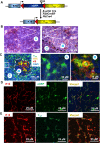
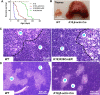
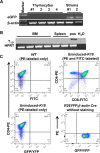
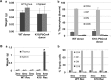
Thymic epithelial cells (TEC), as part of thymic stroma, provide essential growth factors/cytokines and self-antigens to support T cell development and selection. Deletion of Rb family proteins in adult thymic stroma leads to T cell hyperplasia in vivo. To determine whether deletion of Rb specifically in keratin (K) 18 positive TEC was sufficient for thymocyte hyperplasia, we conditionally inactivated Rb and its family members p107 and p130 in K18+ TEC in genetically engineered mice (TgK18GT121; K18 mice). We found that thymocyte hyperproliferation was induced in mice with Rb inactivation in K18+ TEC, while normal T cell development was maintained; suggesting that inactivation of Rb specifically in K18+ TEC was sufficient and responsible for the phenotype. Transplantation of wild type bone marrow cells into mice with Rb inactivation in K18+ TEC resulted in donor T lymphocyte hyperplasia confirming the non-cell autonomous requirement for Rb proteins in K18+ TEC in regulating T cell proliferation. Our data suggests that thymic epithelial cells play an important role in regulating lymphoid proliferation and thymus size.
Conflict of interest statement
The authors have declared that no competing interests exist. KK, DCH and JK are government contract employees of Leidos Biomedical Research, Inc., who work in the Frederick National Laboratory for Cancer Research (FNLCR). This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Transgenic expression of cyclin D1 in thymic epithelial precursors promotes epithelial and T cell development.J Immunol. 2000 Feb 15;164(4):1881-8. doi: 10.4049/jimmunol.164.4.1881. J Immunol. 2000. PMID: 10657637
-
Abnormal thymic microenvironment in insulin-like growth factor-II transgenic mice.Neuroimmunomodulation. 2005;12(2):100-12. doi: 10.1159/000083582. Neuroimmunomodulation. 2005. PMID: 15785112
-
Altered T cell differentiation and Notch signaling induced by the ectopic expression of keratin K10 in the epithelial cells of the thymus.J Cell Biochem. 2005 Jun 1;95(3):543-58. doi: 10.1002/jcb.20406. J Cell Biochem. 2005. PMID: 15786499
-
Role of thymic stromal cells in thymocyte education: a comparitive analysis of different models.Thymus. 1994;22(4):201-13. Thymus. 1994. PMID: 7985221 Review.
-
The thymus and the immune system: layered levels of control.J Thorac Oncol. 2010 Oct;5(10 Suppl 4):S273-6. doi: 10.1097/JTO.0b013e3181f20474. J Thorac Oncol. 2010. PMID: 20859118 Free PMC article. Review.
Cited by
-
Mesothelioma Mouse Models with Mixed Genomic States of Chromosome and Microsatellite Instability.Cancers (Basel). 2022 Jun 24;14(13):3108. doi: 10.3390/cancers14133108. Cancers (Basel). 2022. PMID: 35804881 Free PMC article.
-
Organoids and metastatic orthotopic mouse model for mismatch repair-deficient colorectal cancer.Front Oncol. 2023 Sep 8;13:1223915. doi: 10.3389/fonc.2023.1223915. eCollection 2023. Front Oncol. 2023. PMID: 37746286 Free PMC article.
References
-
- Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40(5):403–39. Epub 2002/05/16. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

