Evaluation of the Clinical and Microbiological Response to Salmonella Paratyphi A Infection in the First Paratyphoid Human Challenge Model
- PMID: 28158395
- PMCID: PMC5439345
- DOI: 10.1093/cid/cix042
Evaluation of the Clinical and Microbiological Response to Salmonella Paratyphi A Infection in the First Paratyphoid Human Challenge Model
Abstract
Background: To expedite the evaluation of vaccines against paratyphoid fever, we aimed to develop the first human challenge model of Salmonella enterica serovar Paratyphi A infection.
Methods: Two groups of 20 participants underwent oral challenge with S. Paratyphi A following sodium bicarbonate pretreatment at 1 of 2 dose levels (group 1: 1-5 × 103 colony-forming units [CFU] and group 2: 0.5-1 × 103 CFU). Participants were monitored in an outpatient setting with daily clinical review and collection of blood and stool cultures. Antibiotic treatment was started when prespecified diagnostic criteria were met (temperature ≥38°C for ≥12 hours and/or bacteremia) or at day 14 postchallenge.
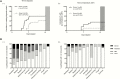
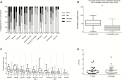
Results: The primary study objective was achieved following challenge with 1-5 × 103 CFU (group 1), which resulted in an attack rate of 12 of 20 (60%). Compared with typhoid challenge, paratyphoid was notable for high rates of subclinical bacteremia (at this dose, 11/20 [55%]). Despite limited symptoms, bacteremia persisted for up to 96 hours after antibiotic treatment (median duration of bacteremia, 53 hours [interquartile range, 24-85 hours]). Shedding of S. Paratyphi A in stool typically preceded onset of bacteremia.
Conclusions: Challenge with S. Paratyphi A at a dose of 1-5 × 103 CFU was well tolerated and associated with an acceptable safety profile. The frequency and persistence of bacteremia in the absence of clinical symptoms was notable, and markedly different from that seen in previous typhoid challenge studies. We conclude that the paratyphoid challenge model is suitable for the assessment of vaccine efficacy using endpoints that include bacteremia and/or symptomatology.
Clinical trials registration: NCT02100397.
Keywords: Salmonella enterica paratyphi A; enteric fever; human challenge study; immune responses; paratyphoid infection.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- McClelland M, Sanderson KE, Clifton SW, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet 2004; 36:1268–74. - PubMed
-
- Levine MM, Ferreccio C, Black RE, Lagos R, San Martin O, Blackwelder WC. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica serovar Paratyphi B. Clin Infect Dis 2007; 45(suppl 1:S24–8. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

