The role of neuroimmune signaling in alcoholism
- PMID: 28159648
- PMCID: PMC5493978
- DOI: 10.1016/j.neuropharm.2017.01.031
The role of neuroimmune signaling in alcoholism
Abstract
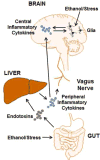
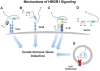
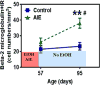
Alcohol consumption and stress increase brain levels of known innate immune signaling molecules. Microglia, the innate immune cells of the brain, and neurons respond to alcohol, signaling through Toll-like receptors (TLRs), high-mobility group box 1 (HMGB1), miRNAs, pro-inflammatory cytokines and their associated receptors involved in signaling between microglia, other glia and neurons. Repeated cycles of alcohol and stress cause a progressive, persistent induction of HMGB1, miRNA and TLR receptors in brain that appear to underlie the progressive and persistent loss of behavioral control, increased impulsivity and anxiety, as well as craving, coupled with increasing ventral striatal responses that promote reward seeking behavior and increase risk of developing alcohol use disorders. Studies employing anti-oxidant, anti-inflammatory, anti-depressant, and innate immune antagonists further link innate immune gene expression to addiction-like behaviors. Innate immune molecules are novel targets for addiction and affective disorders therapies. This article is part of the Special Issue entitled "Alcoholism".
Keywords: Addiction; Alcohol; Azithromycin (PubChem CID: 447043); Cytokines; Glycyrrhizin (PubChem CID: 14982); HMGB1; Ibudilast (PubChem CID: 3671); Indomethacin (PubChem CID: 3715); Minocycline (PubChem CID: 54675783); Naltrexone (PubChem CID: 5360515); Pioglitazone (PubChem CID: 4829); Rapamycin (PubChem CID: 5284616); Rifampin (PubChem CID: 5381226); Simvastatin (PubChem CID: 54454); TLR; miRNA-let-7.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures




References
-
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. - PubMed
-
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

