Myeloproliferative neoplasm stem cells
- PMID: 28159736
- PMCID: PMC5413298
- DOI: 10.1182/blood-2016-10-696005
Myeloproliferative neoplasm stem cells
Abstract
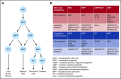
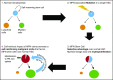
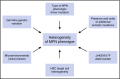
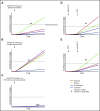
Myeloproliferative neoplasms (MPNs) arise in the hematopoietic stem cell (HSC) compartment as a result of the acquisition of somatic mutations in a single HSC that provides a selective advantage to mutant HSC over normal HSC and promotes myeloid differentiation to engender a myeloproliferative phenotype. This population of somatically mutated HSC, which initiates and sustains MPNs, is termed MPN stem cells. In >95% of cases, mutations that drive the development of an MPN phenotype occur in a mutually exclusive manner in 1 of 3 genes: JAK2, CALR, or MPL The thrombopoietin receptor, MPL, is the key cytokine receptor in MPN development, and these mutations all activate MPL-JAK-STAT signaling in MPN stem cells. Despite common biological features, MPNs display diverse disease phenotypes as a result of both constitutional and acquired factors that influence MPN stem cells, and likely also as a result of heterogeneity in the HSC in which MPN-initiating mutations arise. As the MPN clone expands, it exerts cell-extrinsic effects on components of the bone marrow niche that can favor the survival and expansion of MPN stem cells over normal HSC, further sustaining and driving malignant hematopoiesis. Although developed as targeted therapies for MPNs, current JAK2 inhibitors do not preferentially target MPN stem cells, and as a result, rarely induce molecular remissions in MPN patients. As the understanding of the molecular mechanisms underlying the clonal dominance of MPN stem cells advances, this will help facilitate the development of therapies that preferentially target MPN stem cells over normal HSC.
© 2017 by The American Society of Hematology.
Figures
References
-
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379-2390. - PubMed
-
- Araki M, Yang Y, Masubuchi N, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood. 2016;127(10):1307-1316. - PubMed
-
- Chachoua I, Pecquet C, El-Khoury M, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127(10):1325-1335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

