Tissue Transglutaminase Modulates Vascular Stiffness and Function Through Crosslinking-Dependent and Crosslinking-Independent Functions
- PMID: 28159817
- PMCID: PMC5523743
- DOI: 10.1161/JAHA.116.004161
Tissue Transglutaminase Modulates Vascular Stiffness and Function Through Crosslinking-Dependent and Crosslinking-Independent Functions
Abstract
Background: The structural elements of the vascular wall, namely, extracellular matrix and smooth muscle cells (SMCs), contribute to the overall stiffness of the vessel. In this study, we examined the crosslinking-dependent and crosslinking-independent roles of tissue transglutaminase (TG2) in vascular function and stiffness.
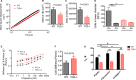
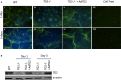
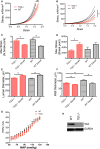
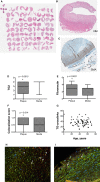
Methods and results: SMCs were isolated from the aortae of TG2-/- and wild-type (WT) mice. Cell adhesion was examined by using electrical cell-substrate impedance sensing and PicoGreen assay. Cell motility was examined using a Boyden chamber assay. Cell proliferation was examined by electrical cell-substrate impedance sensing and EdU incorporation assays. Cell micromechanics were studied using magnetic torsion cytometry and spontaneous nanobead tracer motions. Aortic mechanics were examined by tensile testing. Vasoreactivity was studied by wire myography. SMCs from TG2-/- mice had delayed adhesion, reduced motility, and accelerated de-adhesion and proliferation rates compared with those from WT. TG2-/- SMCs were stiffer and displayed fewer cytoskeletal remodeling events than WT. Collagen assembly was delayed in TG2-/- SMCs and recovered with adenoviral transduction of TG2. Aortic rings from TG2-/- mice were less stiff than those from WT; stiffness was partly recovered by incubation with guinea pig liver TG2 independent of crosslinking function. TG2-/- rings showed augmented response to phenylephrine-mediated vasoconstriction when compared with WT. In human coronary arteries, vascular media and plaque, high abundance of fibronectin expression, and colocalization with TG2 were observed.
Conclusions: TG2 modulates vascular function/tone by altering SMC contractility independent of its crosslinking function and contributes to vascular stiffness by regulating SMC proliferation and matrix remodeling.
Keywords: crosslinking; matrix remodeling; tissue transglutaminase; vascular biology; vascular disease; vascular smooth muscle; vascular stiffness; vessel.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


References
-
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. - PubMed
-
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. - PubMed
-
- Lacolley P, Challande P, Osborne‐Pellegrin M, Regnault V. Genetics and pathophysiology of arterial stiffness. Cardiovasc Res. 2009;81:637–648. - PubMed
-
- Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107:615–619. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

