Can patient involvement improve patient safety? A cluster randomised control trial of the Patient Reporting and Action for a Safe Environment (PRASE) intervention
- PMID: 28159854
- PMCID: PMC5537521
- DOI: 10.1136/bmjqs-2016-005570
Can patient involvement improve patient safety? A cluster randomised control trial of the Patient Reporting and Action for a Safe Environment (PRASE) intervention
Abstract
Objective: To evaluate the efficacy of the Patient Reporting and Action for a Safe Environment intervention.
Design: A multicentre cluster randomised controlled trial.
Setting: Clusters were 33 hospital wards within five hospitals in the UK.
Participants: All patients able to give informed consent were eligible to take part. Wards were allocated to the intervention or control condition.
Intervention: The ward-level intervention comprised two tools: (1) a questionnaire that asked patients about factors contributing to safety (patient measure of safety (PMOS)) and (2) a proforma for patients to report both safety concerns and positive experiences (patient incident reporting tool). Feedback was considered in multidisciplinary action planning meetings.
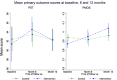
Measurements: Primary outcomes were routinely collected ward-level harm-free care (HFC) scores and patient-level feedback on safety (PMOS).
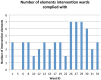
Results: Intervention uptake and retention of wards was 100% and patient participation was high (86%). We found no significant effect of the intervention on any outcomes at 6 or 12 months. However, for new harms (ie, those for which the wards were directly accountable) intervention wards did show greater, though non-significant, improvement compared with control wards. Analyses also indicated that improvements were largest for wards that showed the greatest compliance with the intervention.
Limitations: Adherence to the intervention, particularly the implementation of action plans, was poor. Patient safety outcomes may represent too blunt a measure.
Conclusions: Patients are willing to provide feedback about the safety of their care. However, we were unable to demonstrate any overall effect of this intervention on either measure of patient safety and therefore cannot recommend this intervention for wider uptake. Findings indicate promise for increasing HFC where wards implement ≥75% of the intervention components.
Trial registration number: ISRCTN07689702; pre-results.
Keywords: Cluster trials; Healthcare quality improvement; Patient safety; Patient-centred care; Randomised controlled trial.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- World Health Organization. World alliance for patient safety. Geneva: World Health Organization, 2004.
-
- Thomas EJ, Studdert DM, Burstin HR, et al. . Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care 2000;38:261–71. - PubMed
-
- Wachter RM. Patient safety at ten: unmistakable progress, troubling gaps. Health Aff 2010;29:165–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
