What Are the Most Powerful Immunogen Design Vaccine Strategies? Reverse Vaccinology 2.0 Shows Great Promise
- PMID: 28159875
- PMCID: PMC5540812
- DOI: 10.1101/cshperspect.a030262
What Are the Most Powerful Immunogen Design Vaccine Strategies? Reverse Vaccinology 2.0 Shows Great Promise
Abstract
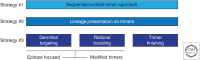
Functional antibodies, i.e., those with antipathogen activity in in vitro assays, are generally the best correlate of vaccine protection. Mimics of natural infection, including live attenuated and killed pathogens, which induce such antibodies in vivo, have generated highly successful vaccines. However, pathogens that induce functional antibodies at lower levels or more sporadically have been more refractory to vaccine design. Such pathogens are being tackled by more systematic approaches involving identifying functional antibodies, templating immunogens from the antibodies, and then evaluating the immunogens iteratively. I believe this is a powerful new approach to vaccine design as discussed below.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Burton DR. 2002. Antibodies, viruses and vaccines. Nat Rev Immunol 2: 706–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
