Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection
- PMID: 28159893
- PMCID: PMC5338410
- DOI: 10.1073/pnas.1621418114
Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection
Erratum in
-
Correction for Mylvaganam et al., Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection.Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):E3366. doi: 10.1073/pnas.1703867114. Epub 2017 Apr 10. Proc Natl Acad Sci U S A. 2017. PMID: 28396390 Free PMC article. No abstract available.
Abstract
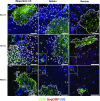
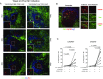
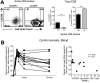
A significant challenge to HIV eradication is the elimination of viral reservoirs in germinal center (GC) T follicular helper (Tfh) cells. However, GCs are considered to be immune privileged for antiviral CD8 T cells. Here, we show a population of simian immunodeficiency virus (SIV)-specific CD8 T cells express CXCR5 (C-X-C chemokine receptor type 5, a chemokine receptor required for homing to GCs) and expand in lymph nodes (LNs) following pathogenic SIV infection in a cohort of vaccinated macaques. This expansion was greater in animals that exhibited superior control of SIV. The CXCR5+ SIV-specific CD8 T cells demonstrated enhanced polyfunctionality, restricted expansion of antigen-pulsed Tfh cells in vitro, and possessed a unique gene expression pattern related to Tfh and Th2 cells. The increase in CXCR5+ CD8 T cells was associated with the presence of higher frequencies of SIV-specific CD8 T cells in the GC. Following TCR-driven stimulation in vitro, CXCR5+ but not CXCR5- CD8 T cells generated both CXCR5+ as well as CXCR5- cells. However, the addition of TGF-β to CXCR5- CD8 T cells induced a population of CXCR5+ CD8 T cells, suggesting that this cytokine may be important in modulating these CXCR5+ CD8 T cells in vivo. Thus, CXCR5+ CD8 T cells represent a unique subset of antiviral CD8 T cells that expand in LNs during chronic SIV infection and may play a significant role in the control of pathogenic SIV infection.
Keywords: CXCR5+CD8+ T cells; HIV; SIV; follicular CD8 T cells; lymphoid follicles.
Conflict of interest statement
Conflict of interest statement: R.R.A. is a coinventor of DNA/MVA vaccine technology, and Emory University licensed this technology to Geovax Inc.
Figures







References
-
- Schmitz JE, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–860. - PubMed
-
- Migueles SA, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3(11):1061–1068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

