IL-2 Modulates the TCR Signaling Threshold for CD8 but Not CD4 T Cell Proliferation on a Single-Cell Level
- PMID: 28159902
- PMCID: PMC5340617
- DOI: 10.4049/jimmunol.1601453
IL-2 Modulates the TCR Signaling Threshold for CD8 but Not CD4 T Cell Proliferation on a Single-Cell Level
Abstract
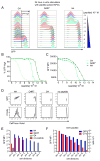
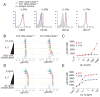
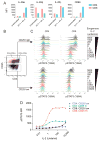
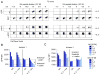
Lymphocytes integrate Ag and cytokine receptor signals to make cell fate decisions. Using a specific reporter of TCR signaling that is insensitive to cytokine signaling, Nur77-eGFP, we identify a sharp, minimal threshold of cumulative TCR signaling required for proliferation in CD4 and CD8 T cells that is independent of both Ag concentration and affinity. Unexpectedly, IL-2 reduces this threshold in CD8 but not CD4 T cells, suggesting that integration of multiple mitogenic inputs may alter the minimal requirement for TCR signaling in CD8 T cells. Neither naive CD4 nor naive CD8 T cells are responsive to low doses of IL-2. We show that activated CD8 T cells become responsive to low doses of IL-2 more quickly than CD4 T cells, and propose that this relative delay in turn accounts for the differential effects of IL-2 on the minimal TCR signaling threshold for proliferation in these populations. In contrast to Nur77-eGFP, c-Myc protein expression integrates mitogenic signals downstream of both IL-2 and the TCR, yet marks an invariant minimal threshold of cumulative mitogenic stimulation required for cell division. Our work provides a conceptual framework for understanding the regulation of clonal expansion of CD8 T cells by subthreshold TCR signaling in the context of mitogenic IL-2 signals, thereby rendering CD8 T cells exquisitely dependent upon environmental cues. Conversely, CD4 T cell proliferation requires an invariant minimal intensity of TCR signaling that is not modulated by IL-2, thereby restricting responses to low-affinity or low-abundance self-antigens even in the context of an inflammatory milieu.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures




References
-
- Marchingo JM, Kan A, Sutherland RM, Duffy KR, Wellard CJ, Belz GT, Lew AM, Dowling MR, Heinzel S, Hodgkin PD. T cell signaling. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science (New York, NY) 2014;346:1123–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

