Leveraging Genomic Factors to Improve Benefit-Risk
- PMID: 28160443
- PMCID: PMC5355972
- DOI: 10.1111/cts.12439
Leveraging Genomic Factors to Improve Benefit-Risk
Figures
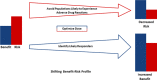
References
-
- Squassina, A. et al Realities and expectations of pharmacogenomics and personalized medicine: Impact of translating genetic knowledge into clinical practice. Pharmacogenomics. 8, 1149–1167 (2010). - PubMed
-
- FDA‐NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource (Internet). Silver Spring (MD): Food and Drug Administration (US); 2016‐. Glossary. 2016. Jan 28 (Updated 2016 Apr 28). Available from: https://www.ncbi.nlm.nih.gov/books/NBK338448/ Co‐published by National Institutes of Health (US), Bethesda (MD). - PubMed
-
- Zineh, I. & Pacanowski, M.A. Pharmacogenomics in the assessment of therapeutic risks versus benefits: Inside the United States Food and Drug Administration. Pharmacotherapy. 8, 729–735 (2011). - PubMed
-
- Pacanowski, M.A. , Leptak, C. & Zineh, I. Next‐generation medicines: Past regulatory experience and considerations for the future. Clin. Pharmacol. Ther. 3, 247–249 (2014). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

