Biophysical and neural basis of resting state functional connectivity: Evidence from non-human primates
- PMID: 28161319
- PMCID: PMC5410395
- DOI: 10.1016/j.mri.2017.01.020
Biophysical and neural basis of resting state functional connectivity: Evidence from non-human primates
Abstract
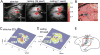
Functional MRI (fMRI) has evolved from simple observations of regional changes in MRI signals caused by cortical activity induced by a task or stimulus, to task-free acquisitions of images in a resting state. Such resting state signals contain low frequency fluctuations which may be correlated between voxels, and strongly correlated regions are deemed to reflect functional connectivity within synchronized circuits. Resting state functional connectivity (rsFC) measures have been widely adopted by the neuroscience community, and are being used and interpreted as indicators of intrinsic neural circuits and their functional states in a broad range of applications, both basic and clinical. However, there has been relatively little work reported that validates whether inter-regional correlations in resting state fluctuations of fMRI (rsfMRI) signals actually measure functional connectivity between brain regions, or to establish how MRI data correlate with other metrics of functional connectivity. In this mini-review, we summarize recent studies of rsFC within mesoscopic scale cortical networks (100μm-10mm) within a well defined functional region of primary somatosensory cortex (S1), as well as spinal cord and brain white matter in non-human primates, in which we have measured spatial patterns of resting state correlations and validated their interpretation with electrophysiological signals and anatomic connections. Moreover, we emphasize that low frequency correlations are a general feature of neural systems, as evidenced by their presence in the spinal cord as well as white matter. These studies demonstrate the valuable role of high field MRI and invasive measurements in an animal model to inform the interpretation of human imaging studies.
Keywords: Cortex; Monkey; Neuroimaging; Resting state; Spinal cord; White matter; fMRI.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
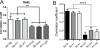




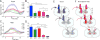



References
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. - PubMed
-
- Guye M, Bartolomei F, Ranjeva JP. Imaging structural and functional connectivity: towards a unified definition of human brain organization? Curr Opin Neurol. 2008;21(4):393–403. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

