Memory B Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis
- PMID: 28161400
- PMCID: PMC5474520
- DOI: 10.1016/j.ebiom.2017.01.042
Memory B Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis
Abstract
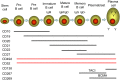
Although multiple sclerosis (MS) is considered to be a CD4, Th17-mediated autoimmune disease, supportive evidence is perhaps circumstantial, often based on animal studies, and is questioned by the perceived failure of CD4-depleting antibodies to control relapsing MS. Therefore, it was interestingly to find that current MS-treatments, believed to act via T cell inhibition, including: beta-interferons, glatiramer acetate, cytostatic agents, dimethyl fumarate, fingolimod, cladribine, daclizumab, rituximab/ocrelizumab physically, or functionally in the case of natalizumab, also depleted CD19+, CD27+ memory B cells. This depletion was substantial and long-term following CD52 and CD20-depletion, and both also induced long-term inhibition of MS with few treatment cycles, indicating induction-therapy activity. Importantly, memory B cells were augmented by B cell activating factor (atacicept) and tumor necrosis factor (infliximab) blockade that are known to worsen MS. This creates a unifying concept centered on memory B cells that is consistent with therapeutic, histopathological and etiological aspects of MS.
Keywords: Autoimmunity; Disease modifying treatment; Immunotherapy; Memory B cell; Multiple sclerosis.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Agahozo M.C., Peferoen L., Baker D., Amor S. CD20 therapies in multiple sclerosis and experimental autoimmune encephalomyelitis - targeting T or B cells? Mult. Scler. Relat. Disord. 2016;9:110–117. - PubMed
-
- Alping P., Frisell T., Novakova L. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann. Neurol. 2016;79:950–958. - PubMed
-
- Ascherio A., Munger K.L. EBV and autoimmunity. Curr. Top. Microbiol. Immunol. 2015;390:365–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

