Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals
- PMID: 28161470
- PMCID: PMC5776681
- DOI: 10.1016/j.jhep.2017.01.020
Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals
Abstract
Background & aims: The approval of all-oral direct-acting antiviral (DAA) regimens for the treatment of hepatitis C virus (HCV) has led to the expansion of therapy to include patients with cirrhosis who have hepatocellular carcinoma (HCC). Data on the use of DAAs in HCV+ patients with HCC is limited. The aim of this study was to assess the efficacy of all-oral-DAA regimens in HCV+ cirrhotic patients who have or had HCC compared to those without HCC.
Methods: A retrospective cohort study was conducted on all cirrhotic patients who were treated for HCV with DAAs at our institution between January 2014 and November 2015.
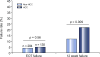
Results: A total of 421 HCV+ patients with cirrhosis were identified, of whom 33% had active or a history of HCC. Failure to achieve sustained virologic response (SVR) occurred in 21% of patients with HCC compared to 12% of patients without HCC (p=0.009). Of the 29 patients with HCC who did not achieve SVR, 27 (93%) occurred when an active tumor was present. DAA therapy in the presence of an inactive tumor or after removal of tumor (resection/transplant) resulted in excellent SVR rates, similar to those without HCC (p<0.0001). In multivariable analysis, the primary predictor of DAA treatment failure was the presence of active HCC at the time of HCV treatment initiation (adjusted odds ratio=8.5, 95% confidence interval=3.90-18.49).
Conclusions: The presence of active HCC tumor at the initiation of HCV therapy is significantly associated with all-oral DAA treatment failure. HCV treatment after curative therapies for HCC resulted in excellent SVR.
Lay summary: The new medications for hepatitis C have excellent cure rates. However, our study shows that in patients with both liver cancer and hepatitis C, they do not achieve these cure rates. Patients with liver cancer are almost 8 times more likely to fail hepatitis C treatment than patients without liver cancer.
Keywords: Cirrhosis; Hepatitis C virus; Hepatocellular carcinoma; Liver cancer; Liver transplant; Sustained viral response; Treatment.
Copyright © 2017 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Prenner: Nothing to disclose. VanWagner: Research: Novartis; Speaker’s Bureau: Salix. Flamm: Research: Gilead, Abbvie; Speaker’s Bureau: Gilead, Abbvie, Merck. Advisory Boards: Gilead, Abbvie, Merck. Salem: Consultant: BTG, Merit, Boston Scientific, Amgen, Terumo. Lewandowski: Advisory Board: BTG, Boston Scientific. Consultant: Cook Medical. Kulik: Advisory Boards: Gilead, Baylor, Salix. For full details, please see the supplementary disclosures file.
Figures

Similar articles
-
Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis.J Hepatol. 2019 Sep;71(3):473-485. doi: 10.1016/j.jhep.2019.04.017. Epub 2019 May 13. J Hepatol. 2019. PMID: 31096005
-
Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients.J Hepatol. 2019 Aug;71(2):265-273. doi: 10.1016/j.jhep.2019.03.027. Epub 2019 Apr 6. J Hepatol. 2019. PMID: 30959157
-
Hepatocellular Carcinoma after Achievement of Sustained Viral Response with Daclatasvir and Asunaprevir in Patients with Chronic Hepatitis C Virus Infection.Dig Dis. 2017;35(6):565-573. doi: 10.1159/000480183. Epub 2017 Oct 17. Dig Dis. 2017. PMID: 29040989
-
Long-term effect of sustained virological response on hepatocellular carcinoma in patients with hepatitis C in Canada.J Hepatol. 2017 Mar;66(3):504-513. doi: 10.1016/j.jhep.2016.10.028. Epub 2016 Nov 4. J Hepatol. 2017. PMID: 27818234
-
The impact of treatment of hepatitis C with DAAs on the occurrence of HCC.Liver Int. 2018 Feb;38 Suppl 1:139-145. doi: 10.1111/liv.13659. Liver Int. 2018. PMID: 29427487 Review.
Cited by
-
AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review.Gastroenterology. 2019 Jun;156(8):2149-2157. doi: 10.1053/j.gastro.2019.02.046. Epub 2019 Mar 13. Gastroenterology. 2019. PMID: 30878469 Free PMC article. Review.
-
Hepatocellular carcinoma or interferon-based therapy history attenuates sofosbuvir/ribavirin for Japanese genotype 2 hepatitis C virus.World J Gastroenterol. 2018 Apr 7;24(13):1478-1485. doi: 10.3748/wjg.v24.i13.1478. World J Gastroenterol. 2018. PMID: 29632428 Free PMC article.
-
Direct-acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout.Hepatology. 2018 Aug;68(2):449-461. doi: 10.1002/hep.29855. Epub 2018 May 16. Hepatology. 2018. PMID: 29476694 Free PMC article.
-
Regulation of the Interferon Response by lncRNAs in HCV Infection.Front Microbiol. 2018 Feb 16;9:181. doi: 10.3389/fmicb.2018.00181. eCollection 2018. Front Microbiol. 2018. PMID: 29503633 Free PMC article. Review.
-
Hepatitis C Virus and Hepatocellular Carcinoma: A Narrative Review.J Clin Transl Hepatol. 2018 Mar 28;6(1):79-84. doi: 10.14218/JCTH.2017.00067. Epub 2017 Dec 17. J Clin Transl Hepatol. 2018. PMID: 29607308 Free PMC article. Review.
References
-
- Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516 e501. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Bailly F, Pradat P, Virlogeux V, Zoulim F. Antiviral therapy in patients with hepatitis C virus-induced cirrhosis. Dig Dis. 2015;33:613–623. - PubMed
-
- Majumdar A, Kitson MT, Roberts SK. Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016;43:1276–1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

