Covalent growth factor tethering to direct neural stem cell differentiation and self-organization
- PMID: 28161574
- PMCID: PMC5410182
- DOI: 10.1016/j.actbio.2017.01.068
Covalent growth factor tethering to direct neural stem cell differentiation and self-organization
Abstract
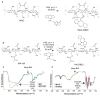
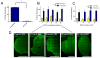
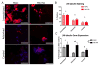
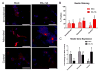
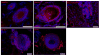
Tethered growth factors offer exciting new possibilities for guiding stem cell behavior. However, many of the current methods present substantial drawbacks which can limit their application and confound results. In this work, we developed a new method for the site-specific covalent immobilization of azide-tagged growth factors and investigated its utility in a model system for guiding neural stem cell (NSC) behavior. An engineered interferon-γ (IFN-γ) fusion protein was tagged with an N-terminal azide group, and immobilized to two different dibenzocyclooctyne-functionalized biomimetic polysaccharides (chitosan and hyaluronan). We successfully immobilized azide-tagged IFN-γ under a wide variety of reaction conditions, both in solution and to bulk hydrogels. To understand the interplay between surface chemistry and protein immobilization, we cultured primary rat NSCs on both materials and showed pronounced biological effects. Expectedly, immobilized IFN-γ increased neuronal differentiation on both materials. Expression of other lineage markers varied depending on the material, suggesting that the interplay of surface chemistry and protein immobilization plays a large role in nuanced cell behavior. We also investigated the bioactivity of immobilized IFN-γ in a 3D environment in vivo and found that it sparked the robust formation of neural tube-like structures from encapsulated NSCs. These findings support a wide range of potential uses for this approach and provide further evidence that adult NSCs are capable of self-organization when exposed to the proper microenvironment.
Statement of significance: For stem cells to be used effectively in regenerative medicine applications, they must be provided with the appropriate cues and microenvironment so that they integrate with existing tissue. This study explores a new method for guiding stem cell behavior: covalent growth factor tethering. We found that adding an N-terminal azide-tag to interferon-γ enabled stable and robust Cu-free 'click' immobilization under a variety of physiologic conditions. We showed that the tagged growth factors retained their bioactivity when immobilized and were able to guide neural stem cell lineage commitment in vitro. We also showed self-organization and neurulation from neural stem cells in vivo. This approach will provide another tool for the orchestration of the complex signaling events required to guide stem cell integration.
Keywords: Central nervous system regeneration; Neural stem cells; Neuroepithelium; Protein immobilization; Strain-promoted alkyne-azide cycloaddition.
Copyright © 2017 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures
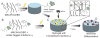

References
-
- Sarhan F, Saif D, Saif A. An overview of traumatic spinal cord injury: part 1. aetiology and pathophysiology. British Journal of Neuroscience Nursing. 2012;8(6):319–325.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

