Long-term safety and efficacy of Omnitrope® in adults with growth hormone deficiency: Italian interim analysis of the PATRO Adults study
- PMID: 28161880
- PMCID: PMC5443881
- DOI: 10.1007/s40618-016-0604-8
Long-term safety and efficacy of Omnitrope® in adults with growth hormone deficiency: Italian interim analysis of the PATRO Adults study
Erratum in
-
Erratum to: Long-term safety and efficacy of Omnitrope® in adults with growth hormone deficiency: Italian interim analysis of the PATRO Adults study.J Endocrinol Invest. 2017 Jun;40(6):679-681. doi: 10.1007/s40618-017-0664-4. J Endocrinol Invest. 2017. PMID: 28421564 Free PMC article. No abstract available.
Abstract
Purpose: To report the long-term effectiveness and safety of the recombinant human growth hormone Omnitrope®, a somatropin biosimilar to Genotropin®, in Italian patients with growth hormone deficiency (GHD) enrolled in the PATRO Adults study.
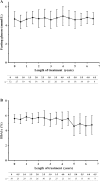
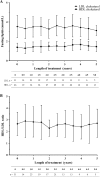
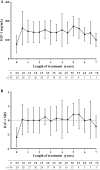
Methods: The PATRO Adults study is an ongoing observational, longitudinal, non-interventional global post-marketing surveillance study, conducted in several European countries. The primary endpoint is long-term safety; secondary endpoints include the effectiveness of Omnitrope®, which was assessed using serum insulin-like growth factor-1 levels, body composition, bone mineral density and lipid levels. Here we report the data from the Italian patients enrolled in the study.
Results: Sixty-seven patients (mean age 50.4 years, 61.2% male) have been enrolled and have received a mean 45.4 ± 24.3 months of Omnitrope®. A total of 55.2% of patients were reported to have experienced adverse events (AEs), including arthralgia, myalgia, abdominal distension and hypoaesthesia, and 4.5% had adverse drug reactions. Fourteen serious AEs have been recorded; none of these are considered related to the study drug. The effectiveness of Omnitrope® was similar to other available somatropin preparations.
Conclusions: This study confirms the effectiveness and safety of Omnitrope® in adult patients with GHD in Italy. However, due to the limited size of the study population, these results need to be further confirmed by the global PATRO Adults study.
Keywords: Growth hormone deficiency; Hypopituitarism; Insulin-like growth factor-1; Omnitrope®; Recombinant human growth hormone; Safety.
Conflict of interest statement
P. Beck-Peccoz is a member of the PATRO Adults Monitoring Committee supported by Sandoz; E. Zecchi and A. Pietropoli are employees of Sandoz; all the other Authors declare no conflicts of interest.
Figures
References
-
- Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157(6):695–700. doi: 10.1530/EJE-07-0631. - DOI - PubMed
-
- Boschetti M, Agosti S, Albanese V, Casalino L, Teti C, Bezante GP, Brunelli C, Albertelli M, Ferone D. One-year GH replacement therapy reduces early cardiac target organ damage (TOD) in adult GHD patients. Endocr. 2016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

