The Pathway From Genes to Gene Therapy in Glaucoma: A Review of Possibilities for Using Genes as Glaucoma Drugs
- PMID: 28161916
- PMCID: PMC6005701
- DOI: 10.22608/APO.2016126
The Pathway From Genes to Gene Therapy in Glaucoma: A Review of Possibilities for Using Genes as Glaucoma Drugs
Abstract
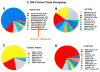
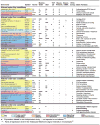
Treatment of diseases with gene therapy is advancing rapidly. The use of gene therapy has expanded from the original concept of re-placing the mutated gene causing the disease to the use of genes to con-trol nonphysiological levels of expression or to modify pathways known to affect the disease. Genes offer numerous advantages over conventional drugs. They have longer duration of action and are more specific. Genes can be delivered to the target site by naked DNA, cells, nonviral, and viral vectors. The enormous progress of the past decade in molecular bi-ology and delivery systems has provided ways for targeting genes to the intended cell/tissue and safe, long-term vectors. The eye is an ideal organ for gene therapy. It is easily accessible and it is an immune-privileged site. Currently, there are clinical trials for diseases affecting practically every tissue of the eye, including those to restore vision in patients with Leber congenital amaurosis. However, the number of eye trials compared with those for systemic diseases is quite low (1.8%). Nevertheless, judg-ing by the vast amount of ongoing preclinical studies, it is expected that such number will increase considerably in the near future. One area of great need for eye gene therapy is glaucoma, where a long-term gene drug would eliminate daily applications and compliance issues. Here, we review the current state of gene therapy for glaucoma and the possibilities for treating the trabecular meshwork to lower intraocular pressure and the retinal ganglion cells to protect them from neurodegeneration.
Keywords: clinical trials; gene therapy; glaucoma; retinal ganglion cells; trabecular meshwork.
Copyright© 2017 Asia-Pacific Academy of Ophthalmology.
Conflict of interest statement
The author has no conflicts of interest to declare.
Figures



References
-
- Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414. - PubMed
-
- Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. - PubMed
-
- Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. - PubMed
-
- Mori K, Gehlbach P, Ando A, et al. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002;43:2428–2434. - PubMed
-
- Campochiaro PA, Nguyen QD, Shah SM, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

