Cell and Growth Factor-Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss in a Mouse Model
- PMID: 28162053
- PMCID: PMC6916118
- DOI: 10.1089/ten.TEA.2016.0457
Cell and Growth Factor-Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss in a Mouse Model
Abstract
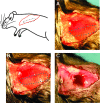
Wounds to the head, neck, and extremities have been estimated to account for ∼84% of reported combat injuries to military personnel. Volumetric muscle loss (VML), defined as skeletal muscle injuries in which tissue loss results in permanent functional impairment, is common among these injuries. The present standard of care entails the use of muscle flap transfers, which suffer from the need for additional surgery when using autografts or the risk of rejection when cadaveric grafts are used. Tissue engineering (TE) strategies for skeletal muscle repair have been investigated as a means to overcome current therapeutic limitations. In that regard, human hair-derived keratin (KN) biomaterials have been found to possess several favorable properties for use in TE applications and, as such, are a viable candidate for use in skeletal muscle repair. Herein, KN hydrogels with and without the addition of skeletal muscle progenitor cells (MPCs) and/or insulin-like growth factor 1 (IGF-1) and/or basic fibroblast growth factor (bFGF) were implanted in an established murine model of surgically induced VML injury to the latissimus dorsi (LD) muscle. Control treatments included surgery with no repair (NR) as well as implantation of bladder acellular matrix (BAM). In vitro muscle contraction force was evaluated at two months postsurgery through electrical stimulation of the explanted LD in an organ bath. Functional data indicated that implantation of KN+bFGF+IGF-1 (n = 8) enabled a greater recovery of contractile force than KN+bFGF (n = 8)***, KN+MPC (n = 8)**, KN+MPC+bFGF+IGF-1 (n = 8)**, BAM (n = 8)*, KN+IGF-1 (n = 8)*, KN+MPCs+bFGF (n = 9)*, or NR (n = 9)**, (*p < 0.05, **p < 0.01, ***p < 0.001). Consistent with the physiological findings, histological evaluation of retrieved tissue revealed much more extensive new muscle tissue formation in groups with greater functional recovery (e.g., KN+IGF-1+bFGF) when compared with observations in tissue from groups with lower functional recovery (i.e., BAM and NR). Taken together, these findings further indicate the general utility of KN biomaterials in TE and, moreover, specifically highlight their potential application in the treatment of VML injuries.
Keywords: FGF; IGF; keratin hydrogel; myogenesis; volumetric muscle loss.
Conflict of interest statement
No competing financial interests exist.
Figures






References
-
- Grogan B.F., and Hsu J.R.; Skeletal Trauma Research Consortium. Volumetric muscle loss. J Am Acad Orthop Surg 19, S35, 2011 - PubMed
-
- Covey D.C. Blast and fragment injuries of the musculoskeletal system. J Bone Joint Surg Am 84-A, 1221, 2002 - PubMed
-
- Masini B.D., Waterman S.M., Wenke J.C., Owens B.D., Hsu J.R., and Ficke J.R. Resource utilization and disability outcome assessment of combat casualties from operation Iraqi Freedom and operation enduring freedom. J Orthop Trauma 23, 261, 2009 - PubMed
-
- Owens B.D., Kragh J.F., Jr., Wenke J.C., Macaitis J., Wade C.E., and Holcomb J.B. Combat wounds in operation Iraqi freedom and operation enduring freedom. J Trauma 64, 295–299, 2008 - PubMed
-
- Thiele O.C., Seeberger R., Engel M., Freier K., and Hoffmann J. Development of the clinical use of distant flaps for head and neck reconstruction. J Craniomaxillofac Surg 42, 79, 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

