Evaluating the impact of targeting livestock for the prevention of human and animal trypanosomiasis, at village level, in districts newly affected with T. b. rhodesiense in Uganda
- PMID: 28162093
- PMCID: PMC5292814
- DOI: 10.1186/s40249-016-0224-8
Evaluating the impact of targeting livestock for the prevention of human and animal trypanosomiasis, at village level, in districts newly affected with T. b. rhodesiense in Uganda
Abstract
Background: Uganda has suffered from a series of epidemics of Human African Trypanosomiasis (HAT), a tsetse transmitted disease, also known as sleeping sickness. The area affected by acute Trypanosoma brucei rhodesiense HAT (rHAT) has been expanding, driven by importation of infected cattle into regions previously free of the disease. These regions are also affected by African Animal Trypanosomiasis (AAT) demanding a strategy for integrated disease control.
Methods: In 2008, the Public Private Partnership, Stamp Out Sleeping Sickness (SOS) administered a single dose of trypanocide to 31 486 head of cattle in 29 parishes in Dokolo and Kaberamaido districts. This study examines the impact of this intervention on the prevalence of rHAT and AAT trypanosomes in cattle from villages that had (HAT+ve) or had not (HAT-ve) experienced a recent case of rHAT. Cattle herds from 20 villages were sampled and screened by PCR, pre-intervention and 6-months post-intervention, for the presence or absence of: Trypanosoma brucei s.l.; human infective T. b. rhodesiense; Trypanosoma vivax; and Trypanosoma congolense savannah.
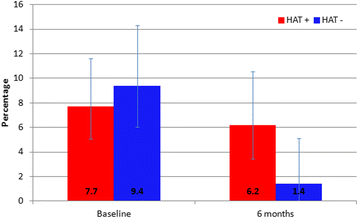
Results: Post-intervention, there was a significant decrease in the prevalence of T. brucei s.l. and the human infective sub-species T. b. rhodesiense in village cattle across all 20 villages. The prevalence of T. b. rhodesiense was reduced from 2.4% to 0.74% (P < 0.0001), with the intervention showing greater impact in HAT-ve villages. The number of villages containing cattle harbouring human infective parasites decreased from 15/20 to 8/20, with T. b. rhodesiense infection mainly persisting within cattle in HAT+ve villages (six/eight). The proportion of T. brucei s.l. infections identified as human infective T. b. rhodesiense decreased after the intervention from 8.3% (95% CI = 11.1-5.9%) to 4.1% (95% CI = 6.8-2.3%). Villages that had experienced a recent human case (HAT+ve villages) showed a significantly higher prevalence for AAT both pre- and post-intervention. For AAT the prevalence of T. vivax was significantly reduced from 5.9% to 0.05% post-intervention while the prevalence of T. congolense increased from 8.0% to 12.2%.
Conclusions: The intervention resulted in a significant decrease in the prevalence of T. brucei s.l., human infective T. b. rhodesiense and T. vivax infection in village cattle herds. The proportion of T. brucei s.l. that were human infective, decreased from 1:12 T. brucei s.l. infections before the intervention to 1:33 post-intervention. It is clearly more difficult to eliminate T. b. rhodesiense from cattle in villages that have experienced a human case. Evidence of elevated levels of AAT in livestock within village herds is a useful indicator of risk for rHAT in Uganda. Integrated veterinary and medical surveillance is key to successful control of zoonotic rHAT.
Keywords: African animal trypanosomiasis (AAT); Human African trypanosomiasis (HAT); Sleeping sickness; T. b. brucei; T. b. rhodesiense; Trypanosma brucei rhodesiense HAT (rHAT); Uganda.
Figures





References
-
- Fèvre EM, Coleman PG, Welburn SC. Maudlin I Reanalyzing the 1900–1920 sleeping sickness epidemic in Uganda. Emerg Infect Dis. 2004;10(4):567–73. - PubMed
-
- Welburn SC, Molyneux DH. Maudlin I Beyond tsetse – implications for research and control of human African trypanosomiasis epidemics. Trends Parasitol. 2016;32(3):230–41. - PubMed
-
- Odiit M, Coleman PG, McDermott JJ, Fevre EM, Welburn SC, Woolhouse MEJ. Spatial and temporal risk factors for the early detection of Trypanosoma brucei rhodesiense sleeping sickness patients in Tororo and Busia districts, Uganda. Trans Roy Soc Trop Med Hyg. 2004;98:569–76. doi: 10.1016/j.trstmh.2003.12.012. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

