Synthetic Pot: Not Your Grandfather's Marijuana
- PMID: 28162792
- PMCID: PMC5329767
- DOI: 10.1016/j.tips.2016.12.003
Synthetic Pot: Not Your Grandfather's Marijuana
Abstract
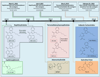
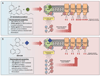
In the early 2000s in Europe and shortly thereafter in the USA, it was reported that 'legal' forms of marijuana were being sold under the name K2 and/or Spice. Active ingredients in K2/Spice products were determined to be synthetic cannabinoids (SCBs), producing psychotropic actions via CB1 cannabinoid receptors, similar to those of Δ9-tetrahydrocannabinol (Δ9-THC), the primary active constituent in marijuana. Often abused by adolescents and military personnel to elude detection in drug tests due to their lack of structural similarity to Δ9-THC, SCBs are falsely marketed as safe marijuana substitutes. Instead, SCBs are a highly structural diverse group of compounds, easily synthesized, which produce very dangerous adverse effects occurring by, as of yet, unknown mechanisms. Therefore, available evidence indicates that K2/Spice products are clearly not safe marijuana alternatives.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no significant conflicts of interest to declare.
Figures


References
-
- Gurney SM, et al. Pharmacology, Toxicology, and Adverse Effects of Synthetic Cannabinoid Drugs. Forensic Science Review. 2014;26(1):53–78. - PubMed
-
- DEA Office of Diversion Control. Department of Justice. 2016 http://www.deadiversion.usdoj.gov/
-
- Zawilska JB, Wojcieszak J. Spice/K2 drugs--more than innocent substitutes for marijuana. Int J Neuropsychopharmacol. 2014;17(3):509–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

