Munc18b Increases Insulin Granule Fusion, Restoring Deficient Insulin Secretion in Type-2 Diabetes Human and Goto-Kakizaki Rat Islets with Improvement in Glucose Homeostasis
- PMID: 28163042
- PMCID: PMC5474508
- DOI: 10.1016/j.ebiom.2017.01.030
Munc18b Increases Insulin Granule Fusion, Restoring Deficient Insulin Secretion in Type-2 Diabetes Human and Goto-Kakizaki Rat Islets with Improvement in Glucose Homeostasis
Abstract
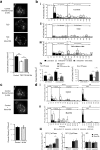
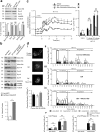
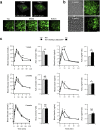
Reduced pancreatic islet levels of Munc18a/SNARE complex proteins have been postulated to contribute to the deficient glucose-stimulated insulin secretion (GSIS) in type-2 diabetes (T2D). Whereas much previous work has purported Munc18a/SNARE complex (Syntaxin-1A/VAMP-2/SNAP25) to be primarily involved in predocked secretory granule (SG) fusion, less is known about newcomer SGs that undergo minimal docking time at the plasma membrane before fusion. Newcomer SG fusion has been postulated to involve a distinct SM/SNARE complex (Munc18b/Syntaxin-3/VAMP8/SNAP25), whose levels we find also reduced in islets of T2D humans and T2D Goto-Kakizaki (GK) rats. Munc18b overexpression by adenovirus infection (Ad-Munc18b), by increasing assembly of Munc18b/SNARE complexes, mediated increased fusion of not only newcomer SGs but also predocked SGs in T2D human and GK rat islets, resulting in rescue of the deficient biphasic GSIS. Infusion of Ad-Munc18b into GK rat pancreas led to sustained improvement in glucose homeostasis. However, Munc18b overexpression in normal islets increased only newcomer SG fusion. Therefore, Munc18b could potentially be deployed in human T2D to rescue the deficient GSIS.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Syntaxin 2 Acts as Inhibitory SNARE for Insulin Granule Exocytosis.Diabetes. 2017 Apr;66(4):948-959. doi: 10.2337/db16-0636. Epub 2017 Jan 23. Diabetes. 2017. PMID: 28115395 Free PMC article.
-
Munc18c mediates exocytosis of pre-docked and newcomer insulin granules underlying biphasic glucose stimulated insulin secretion in human pancreatic beta-cells.Mol Metab. 2015 Feb 16;4(5):418-26. doi: 10.1016/j.molmet.2015.02.004. eCollection 2015 May. Mol Metab. 2015. PMID: 25973389 Free PMC article.
-
Syntaxin-4 mediates exocytosis of pre-docked and newcomer insulin granules underlying biphasic glucose-stimulated insulin secretion in human pancreatic beta cells.Diabetologia. 2015 Jun;58(6):1250-9. doi: 10.1007/s00125-015-3545-4. Epub 2015 Mar 13. Diabetologia. 2015. PMID: 25762204
-
Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis.Diabetes Obes Metab. 2017 Sep;19 Suppl 1:115-123. doi: 10.1111/dom.13001. Diabetes Obes Metab. 2017. PMID: 28880475 Review.
-
Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4.Am J Physiol Regul Integr Comp Physiol. 2010 Mar;298(3):R517-31. doi: 10.1152/ajpregu.00597.2009. Epub 2010 Jan 6. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20053958 Free PMC article. Review.
Cited by
-
Plasma membrane flipping of Syntaxin-2 regulates its inhibitory action on insulin granule exocytosis.Nat Commun. 2022 Oct 31;13(1):6512. doi: 10.1038/s41467-022-33986-3. Nat Commun. 2022. PMID: 36316316 Free PMC article.
-
Characteristics of Berberine Effects on Insulin Exocytosis in Pancreatic β Cells.FASEB J. 2025 Jun 15;39(11):e70718. doi: 10.1096/fj.202501267R. FASEB J. 2025. PMID: 40497406 Free PMC article.
-
Pancreas-specific SNAP23 depletion prevents pancreatitis by attenuating pathological basolateral exocytosis and formation of trypsin-activating autolysosomes.Autophagy. 2021 Oct;17(10):3068-3081. doi: 10.1080/15548627.2020.1852725. Epub 2020 Dec 7. Autophagy. 2021. PMID: 33213278 Free PMC article.
-
Kv2.1 clusters on β-cell plasma membrane act as reservoirs that replenish pools of newcomer insulin granule through their interaction with syntaxin-3.J Biol Chem. 2018 May 4;293(18):6893-6904. doi: 10.1074/jbc.RA118.002703. Epub 2018 Mar 16. J Biol Chem. 2018. PMID: 29549124 Free PMC article.
-
Recent Insights into Beta-cell Exocytosis in Type 2 Diabetes.J Mol Biol. 2020 Mar 6;432(5):1310-1325. doi: 10.1016/j.jmb.2019.12.012. Epub 2019 Dec 19. J Mol Biol. 2020. PMID: 31863749 Free PMC article. Review.
References
-
- Banks K., Qin T., Liang T., Wang A.J., Gaisano H.Y. Biliopancreatic route for effective viral transduction of pancreatic islets. Pancreas. 2014;43:240–244. - PubMed
-
- Gaddy D.F., Riedel M.J., Bertera S., Kieffer T.J., Robbins P.D. dsAAV8-mediated gene transfer and β-cell expression of IL-4 and β-cell growth factors are capable of reversing early-onset diabetes in NOD mice. Gene Ther. 2012;19:791–799. - PubMed
-
- Kwan E.P., Gaisano H.Y. Rescuing the subprime meltdown in insulin exocytosis in diabetes. Ann. N.Y. Acad. Sci. 2009;1152:154–164. - PubMed
-
- Gaisano H.Y. Here come the newcomers granules, better late than never. Trends Endocrinol. Metab. 2014;25:381–388. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

