Picornaviral polymerase structure, function, and fidelity modulation
- PMID: 28163093
- PMCID: PMC5476519
- DOI: 10.1016/j.virusres.2017.01.026
Picornaviral polymerase structure, function, and fidelity modulation
Abstract
Like all positive strand RNA viruses, the picornaviruses replicate their genomes using a virally encoded RNA-dependent RNA polymerase enzyme known as 3Dpol. Over the past decade we have made tremendous advances in our understanding of 3Dpol structure and function, including the discovery of a novel mechanism for closing the active site that allows these viruses to easily fine tune replication fidelity and quasispecies distributions. This review summarizes current knowledge of picornaviral polymerase structure and how the enzyme interacts with RNA and other viral proteins to form stable and processive elongation complexes. The picornaviral RdRPs are among the smallest viral polymerases, but their fundamental molecular mechanism for catalysis appears to be generally applicable as a common feature of all positive strand RNA virus polymerases.
Keywords: Picornavirus; Polymerase; Positive strand RNA virus; RNA-dependent RNA polymerase; Structure.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures


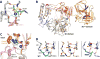


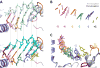

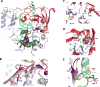
Similar articles
-
Structure-function relationships underlying the replication fidelity of viral RNA-dependent RNA polymerases.J Virol. 2015 Jan;89(1):275-86. doi: 10.1128/JVI.01574-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320316 Free PMC article.
-
Picornavirus RNA polyadenylation by 3D(pol), the viral RNA-dependent RNA polymerase.Virus Res. 2015 Aug 3;206:3-11. doi: 10.1016/j.virusres.2014.12.030. Epub 2015 Jan 3. Virus Res. 2015. PMID: 25559071 Free PMC article. Review.
-
Picornaviral polymerase domain exchanges reveal a modular basis for distinct biochemical activities of viral RNA-dependent RNA polymerases.J Biol Chem. 2020 Jul 31;295(31):10624-10637. doi: 10.1074/jbc.RA120.013906. Epub 2020 Jun 3. J Biol Chem. 2020. PMID: 32493771 Free PMC article.
-
Picornavirus RNA-dependent RNA polymerase.Int J Biochem Cell Biol. 2009 Mar;41(3):498-502. doi: 10.1016/j.biocel.2008.03.019. Epub 2008 Apr 7. Int J Biochem Cell Biol. 2009. PMID: 18487072 Review.
-
Non-template functions of viral RNA in picornavirus replication.Curr Opin Virol. 2011 Nov;1(5):339-46. doi: 10.1016/j.coviro.2011.09.005. Curr Opin Virol. 2011. PMID: 22140418 Free PMC article. Review.
Cited by
-
Residues within the Foot-and-Mouth Disease Virus 3Dpol Nuclear Localization Signal Affect Polymerase Fidelity.J Virol. 2020 Aug 17;94(17):e00833-20. doi: 10.1128/JVI.00833-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581111 Free PMC article.
-
Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design.Nat Rev Mol Cell Biol. 2022 Jan;23(1):21-39. doi: 10.1038/s41580-021-00432-z. Epub 2021 Nov 25. Nat Rev Mol Cell Biol. 2022. PMID: 34824452 Free PMC article. Review.
-
Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites.Antiviral Res. 2020 Jun;178:104793. doi: 10.1016/j.antiviral.2020.104793. Epub 2020 Apr 10. Antiviral Res. 2020. PMID: 32283108 Free PMC article.
-
CoV-er all the bases: Structural perspectives of SARS-CoV-2 RNA synthesis.Enzymes. 2021;49:1-37. doi: 10.1016/bs.enz.2021.06.004. Epub 2021 Aug 23. Enzymes. 2021. PMID: 34696829 Free PMC article. Review.
-
A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase.Nat Commun. 2022 Feb 2;13(1):621. doi: 10.1038/s41467-022-28113-1. Nat Commun. 2022. PMID: 35110538 Free PMC article.
References
-
- Arias A, Arnold JJ, Sierra M, Smidansky ED, Domingo E, Cameron CE. Determinants of RNA-dependent RNA polymerase (in)fidelity revealed by kinetic analysis of the polymerase encoded by a foot-and-mouth disease virus mutant with reduced sensitivity to ribavirin. J Virol. 2008;82:12346–12355. - PMC - PubMed
-
- Arnold JJ, Cameron CE. Poliovirus RNA-dependent RNA polymerase (3D(pol)). Assembly of stable, elongation-competent complexes by using a symmetrical primer-template substrate (sym/sub) J Biol Chem. 2000;275:5329–5336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

